Vered Caplan, CEO of Orgenesis, participated in a Q&A about their therapeutic technology, which converts the liver cells of patients with type 1 diabetes into functioning insulin-producing cells.
Q: How was Orgenesis’ therapeutic technology discovered?
Caplan: In the late 1990s, Professor Sarah Ferber was researching why certain mice were born without a pancreas and uncovered the role of certain factors (known as transcription factors) in the development of pancreatic beta cells. Liver cells and pancreas cells are very closely linked in the fetal development. She found out that in these mice, they were missing a central component that helped develop these cells in the embryo. This component is PDX-1, also known as insulin promoter factor 1. She then used these factors on liver cells and discovered that the liver cells could be actually function as pancreas cells in that they became glucose-responsive, insulin-producing liver cells.
Q: How does Orgenesis’ therapeutic technology work?
Caplan: The Orgenesis approach differs from others in the field because it is focuses on providing a potential cure and enabling the patient to have their own insulin-production and regulation capabilities so they do not have to depend on external insulin injections. With about 15 years of research and development under its belt, Orgenesis has developed a novel therapeutic technology dedicated to converting a patient’s own liver cells into functioning insulin-producing cells.
This ground-breaking, patented technology may provide people with insulin-dependent type 1 diabetes a new means of producing adequate levels of insulin, thereby mitigating or eliminating the need for external insulin injections.
Because liver and pancreas cells are so closely related, adding PDX-1 to liver cells causes a cascade of gene expression that changes or “transdifferentiates” the cells into pancreatic islet-like cells called autologous insulin producing (AIP) cells.
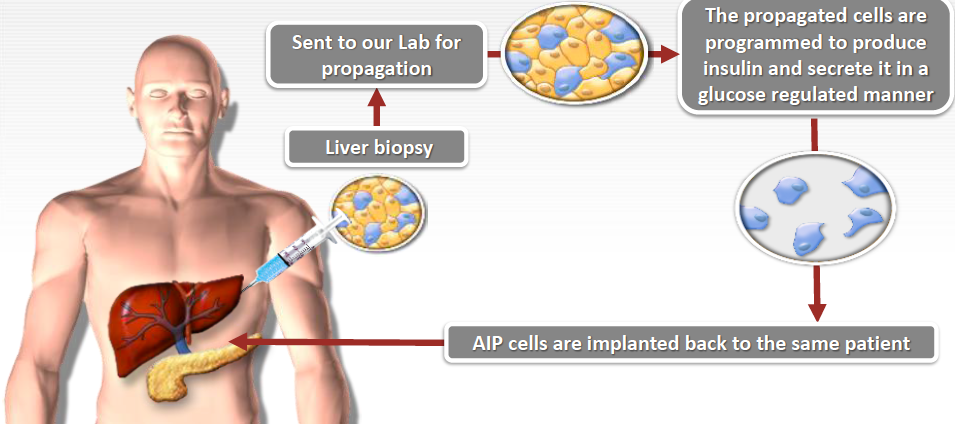
The process involves a patient going into a clinic to have a liver biopsy (a very small amount of liver cells being taken out of the patient’s body). The patient goes home and the cells are sent to a central facility for expansion (the small amount will increase to a large amount) and, with the use of Orgenesis’ technology, are transformed (transdifferentiated) into insulin-producing cells.
This process of expanding and transforming the cells requires unique manufacturing know-how.
Preclinical studies performed in numerous labs worldwide suggest the potential feasibility of converting liver cells to AIP cells. The conversion of liver cells into pancreatic endocrine cells has been completed in numerous animal species and most importantly with human liver cells from about 70 different donors’ aged from 3 to 60 years old.
Q: How could this therapeutic technology potentially cure diabetes?
Caplan: If a patient were provided with a source of insulin-producing cells, they would no longer need insulin injections. The hope is that these AIP cells, when planted in a patient, would replace the need for insulin injections.
The AIP cell approach is unique and highly differentiated when compared to all currently available and development stage alternatives. The AIP cellular technology is being developed as a “practical cure” for patients living with insulin-dependent Type 1 diabetes (T1D). The Juvenile Diabetes Research Foundation (JDRF) defines a “practical cure” as one that provides long-term insulin-independence without the need for associated immunosuppressive therapy, and one that restores a patient’s Quality of Life to (near) normal.
The most commonly used therapy for T1D is insulin replacement with exogenous insulin—administered by either injection or continuously by a pump. Therapeutic procedures like pancreatic islet transplantation, while representing a significant clinical advance, only offer temporary insulin-independence (1-5 years) and require concomitant immunosuppressive therapy. Many other therapies being developed target the early onset or “honeymoon” period of T1D, and seek to slow a patient’s progression to insulin-dependence rather than restore insulin independence.
At Orgenesis, we hope to cure T1D as we know it—not simply enhance the symptomatic management of the disease.
Q: When do you hope for Orgenesis’ therapeutic technology to reach the market? (As, currently, Orgenesis is gearing up for Phase 1 human trials in the U.S. and Europe.)
Caplan: Orgenesis is focused on finding the regulatory pathways that will expedite market entry in the U.S. and throughout the world.
There have been some interesting advancements in certain countries for regenerative medicine recently. For example, the regulatory environment in Japan is such that some regenerative therapies can go to market following successful safety/Phase 1 trials. We still have work to do before we know for sure that our therapy can be offered to the world market, but we are hopeful of steady progress towards this goal.
Q: Do you foresee Orgenesis’ therapeutic technology to be more readily accepted in a particular market? (Specifically, the U.S. vs. Europe.)
Caplan: If we are as successful as we hope, diabetes patients all over the world would have to strongly consider our therapy as an alternative to the treatments they now undergo.
Q: Do you foresee this technology being beneficial for patients with type 2 diabetes? If so/if not, explain.
Caplan: For Type 2 patients who are insulin dependent, a source of new insulin producing cells would be beneficial. We have focused on type 1 diabetes to date, and if we are successful in bringing our therapy to market, our researchers would naturally consider ways to apply our know-how to patients with type 2 diabetes.
Q: Have you seen any other potential treatments for patients with diabetes coming down the pipeline or any trends that manufacturers should be aware of?
Caplan: In a recently published article in The Life Sciences Report, Bernard Siegel, the Executive Director of Genetics Policy Institute (GPI) said, “We are in the middle of a convergence, where breakthroughs in biomaterials, vascularization, tissue engineering, regenerative engineering, and stem cell technologies are joining. For the first time, we are looking at ways to create a blueprint for something that always seemed like an impossible dream. If we could solve the organ shortage by growing tissues, and then making those tissues into organs, it would be a major achievement in alleviation of human suffering.”
Q: What do you foresee for the future of diabetes treatment?
Caplan: There will be more diversity of solutions with more options for patients at different stages of the disease in the future. We foresee new immunotherapies and cellular therapies that could potentially provide practical cures for not only diabetes but also other benefits, such as replacing other types of cells in the body. In the meantime, the management of the disease will improve. A wide diversity of more sophisticated insulin administration technologies, such as inhalable insulin and new, longer-acting insulin will be available. There will also be significant advancements in early diagnosis to treat patients at an earlier stage.
Q: How will manufacturers be affected by these potential changes?
Caplan: The market for diabetes treatment is global and very large. Even if there is a certain percentage of patients in the next few years who can take advantage of a practical cure, there will be a large section of the population who will still need insulin. The impact of new technologies will be gradual—not immediate. As these therapies achieve regulatory approval and become more clinically available, manufacturers will have to diversify their manufacturing line to meet those needs and develop the necessary technologies to provide for these therapies.
Q: Do you have any recommendations for how manufacturers can prepare for the potential production of technologies for type 1 diabetes treatment?
Caplan: The industry is currently undergoing a shift in the approach to developing new therapies. Personalized medicine is at the forefront. In the case of diabetes treatment, manufactures will need to evolve and prepare to make the move from technologies that manufacture recombinant proteins (insulin) to more cellular therapies.
Classic manufacturers in the field have been employing a process that involves leveraging host cells in order to manufacture proteins; the cells themselves are considered a waste product. In cellular therapies, it is the other way around—the cell is the product. And, going forward, personalized medicine will have a greater impact. Each manufacturing lot will be specific to an individual patient. That is a new approach. It involves a new way of looking at manufacturing for each individual patient.
In preparation for this evolution, we at Orgenesis are working with our subsidiary, MaSTherCell on developing good manufacturing practices.
Manufacturing of cells is a sophisticated process. We have also been very fortunate to collaborate with Pall Life Sciences where our scientific teams have been working closely with their development team to utilize their Xpansion™® Multiple Bioreactor. This innovative technology provides Orgenesis with the ability to expand a minimal amount of cells derived from the patient’s own liver into a large amount of cells.
Coming changes in the manufacturing process will be substantial, and we hope to be at the forefront of these changes together with our manufacturing partners.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




