With multiple generations of employees, UPM Pharmaceuticals makes headway in the drug development space, specializing in oral solid dosage forms. Based in Bristol, Tenn., UPM Pharmaceuticals specializes in drug development services from R&D through commercialization, including formulation development, cGMP manufacturing and packaging, analytical method development, testing, and inventory management and distribution services. “We started at…
Filling the Gap
There is an unmet need in the CDMO marketplace, says Avista Pharma Solutions: stand-alone process chemical development support. With 200,000 square feet of laboratory and manufacturing space, contract testing, development, and manufacturing organization Avista Pharma Solutions provides services from early drug development and drug product manufacturing to stand-alone analytical and microbiology testing support for the…
Editor’s Note: Serialization – The Hurry Up and Wait Game
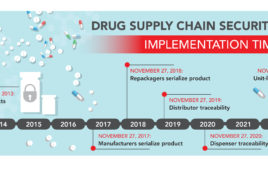
In June, the U.S. FDA extended the Product Identifier Requirements under the Drug Supply Chain Security Act (DSCSA)—resulting in some of the biggest changes in 2017 for manufacturers. The previous implementation timeline dictated that all manufacturers must have serialized products after November 2017. Now, however, manufacturers have a ‘hard’ deadline of November 2018. Until then,…
FDA Drug Shortages: 9/12/17 Update
More Than 200 Potential Jurors Excused from Shkreli’s Trial
The former CEO of Retrophin and Turing Pharmaceuticals, Martin Shkreli—famous for raising the price of Daraprim by 5,000 percent—was convicted of three counts of fraud in federal court earlier this month. According to The New York Times: “[A]fter five days of deliberations, jurors convicted him on three counts of fraud in federal court, and he now…
Experts Comment on FDA’s DSCSA Serialization Extension: Manufacturers Now Have Until 2018
As of June 30, 2017, the U.S. FDA extended the Product Identifier Requirements under the Drug Supply Chain Security Act (DSCSA). The previous implementation timeline dictated that all manufacturers must have serialized products after November 2017. According to the new Guidance document, titled Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance Policy Guidance…
Defining ‘Clean’: Cleanroom Operation Market Trends
AES Clean Technology participates in an exclusive Q&A with Pharmaceutical Processing on cleanroom operations. The pharmaceutical and biotechnology industries, according to Solid State Technology, occupy the second largest slot for cleanroom usage. These rooms, which maintain the concentration of airborne particles, control environmental factors such as air flow patterns, temperature, humidity, cleanliness, and pressurization—among others.…
Continuous Manufacturing: A Practical Industry Solution?
Over the last 50 years, continuous manufacturing technologies have been slow to advance, remaining relatively unchanged. However, there has been an uptick in recent years in the dialogue surrounding continuous manufacturing and its potential applicability in the pharmaceutical and biopharmaceutical industries. At the forefront, the U.S. Food and Drug Administration (FDA) now encourages companies to…
Meeting Niche Market Needs: How a Small CDMO Excels Through Customization
Spanish CDMO IDIFARMA finds home in niche generics and innovative drugs market—excelling in small batch sizes and packaging customization. Founded in 2001, the Spanish contract development and manufacturing organization (CDMO) IDIFARMA was formed to serve the niche generics and innovative drugs market. Targeting the pharmaceutical and biotechnology markets, the company has worked with more than…
Microneedle Technology Makes a Splash in Drug Delivery Space
Every year, companies are coming out with new, innovative drug delivery technologies, improving ease of access and design by modifying the devices for easy administration and the best possible drug absorption. 3M Drug Delivery Systems, a part of the Health Care Business Group of 3M, researches and develops new ways to deliver drugs to patients…