Bristol Myers Squibb announced that it invested $400 million to build a sterile drug product (SDP) facility in Dublin, Ireland. This Cruiserath facility will support the manufacturing and supply of existing medicines and serve as a launch excellence facility for pipeline assets. It marks the first European SDP facility for biologics manufacturing at Bristol Myers…
Takeda wins approval for eosinophilic esophagitis drug Eohilia
Takeda announced this week that it received FDA approval for its Eohilia (budesonide oral suspension) therapy. This makes Eohilia the first and only FDA-approved oral therapy for people 11 years and older with eosinophilic esophagitis (EoE). Takeda plans to offer it in 2 mg/10 mL convenient, single-dose stick packs by the end of February. Eohilia, a…
Catalent cuts 300 jobs ahead of acquisition by Novo Holdings
Catalent said this week that it reduced its headcount by approximately 300 employees as part of a restructuring effort. The company confirmed the workforce reduction in its 10-Q quarterly report, filed on Feb. 14. This also coincides with preparations for an acquisition as Novo Holdings agreed to buy the leading pharmaceutical CDMO for $16.5 billion…
Bora Pharmaceuticals is buying generics maker Upsher-Smith
Bora Pharmaceuticals announced that its board approved the acquisition of Upsher-Smith Laboratories for up to $210 million. Minnesota-based Upsher-Smith features a diversified portfolio of 48 generic products. It has manufacturing facilities in Plymouth and Maple Grove, Minnesota. Taipei, Taiwan-based Bora called the company a reliable partner with solid manufacturing capabilities, a robust distribution network and…
Report: Pharma kicks off year with drug price hikes
Reuters reports that a number of big-name drugmakers, including Pfizer, Sanofi and Takeda plan to raise drug prices to start the year. According to the report, the price hikes apply to more than 500 drugs. Excluding different doses and formulations, the reach extends to more than 140 brands of drugs. Reuters said healthcare research firm 3…
Novo Nordisk to boost GLP-1 capacity with €2.1 billion investment in France
Novo Nordisk announced last week that it plans to invest approximately 16 billion Danish kroner in GLP-1 drug production. That equates to about $2.32 billion or €2.14 billion. The company plans to expand its Chartres, France, production site for its current and future product portfolio within serious chronic diseases. This investment significantly increases the capacity…
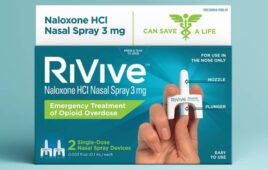
FDA approves another over-the-counter Naloxone nasal spray
Harm Reduction Therapeutics announced that it received FDA approval for its over-the-counter RiVive naloxone nasal spray. Pittsburgh-based HRT designed the 3 mg naloxone HCl nasal spray for the emergency treatment of opioid overdose. Approval helps make free or low-cost OTC nasal spray widely available in the U.S. The FDA first approved Narcan 4 mg OTC…
Sanofi to acquire diabetes treatment developer Provention Bio for $2.9B
Sanofi announced that it agreed to acquire Provention Bio, which develops therapeutics for immune-mediated diseases, for approximately $2.9 billion. Paris, France-based Sanofi agreed to acquire Provention Bio for $25 per share in cash. If the companies complete the acquisition, Sanofi expects a wholly-owned subsidiary to merge with and into Provention Bio. It intends to fund…
Pfizer to acquire Seagen for $43B
Pfizer and Seagen announced today that they entered into an agreement under which Pfizer acquires Seagen for $43 billion. The price of the acquisition comes to $229 in cash per Seagen share, totaling $43 billion. Both companies’ boards unanimously approved the transaction. Seagen develops antibody-drug conjugate (ADC) technology. Four of the 12 total FDA-approved and…
Eli Lilly is cutting the price of insulin by 70%
Eli Lilly (NYSE:LLY) announced today that it plans to execute price reductions of 70% for its most commonly prescribed insulins. Indianapolis-based Lilly also plans to expand its Insulin Value Program. This caps patient out-of-pocket costs at $35 or less per month. The company said it took these actions to “help Americans” in a “complex healthcare…