Bill Cummings discusses Ypsomed and Thinfilm’s new partnership and their approach to non-adherence.
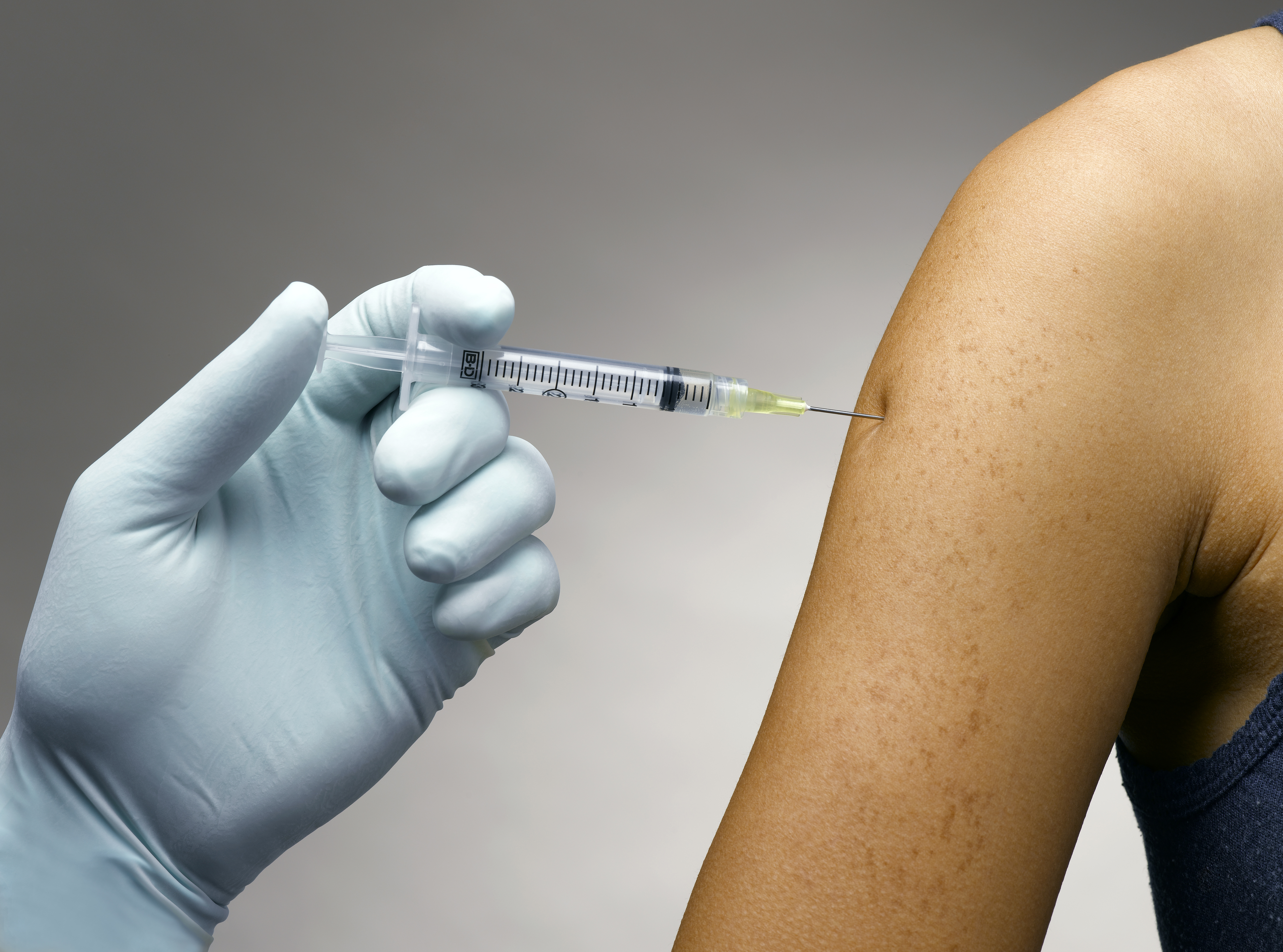
Self-injection medicines are prescribed to millions of people worldwide to help manage various conditions. However, medication adherence is not always a top priority for patients.
According to one study, “non-adherence is a complicated and common problem,” with nearly three out of four Americans reporting that they “do not always take their medicine as directed.”
Consequently, Ypsomed and Thinfilm are striving to change this. They will incorporate smart labels and connectivity to injectable medication—notifying patients of usage guidelines, reminding them of injections, as well as providing refill instructions on their phones.
Researchers may spend years creating and perfecting any one medication, but that has little impact if the patients are not taking medications that they need.
Bill Cummings, Vice President of Marketing and Communications at Thinfilm, participated in a Q&A to talk about the company’s approach to injectables and its new partnership.

Q: There has been a lot of talk about the “Internet of Things.” What is your company’s approach to the IoT?
Cummings: The Internet of Things (IoT) is not just a fad—it’s a growing reality. And more and more it will continue to become a part of our everyday lives as technologies advance and the ecosystem expands. In many respects, however, the extent of the IoT’s reach will ultimately depend on extending beyond the traditional machine-to-machine boundaries to the world of everyday items—disposables, consumables, etc.
In order to diffuse electronic intelligence to flexible packaging, fast moving consumer goods, and other high-volume areas, we’ll need to look for alternatives to traditional silicon-based electronics, which are simply too complex and expensive. At the disposables level, we’re talking about billions—and potentially trillions—of items that are connectable. These are items that only need to be “just smart enough.”
We believe that printed electronics products—with ultra-high volume manufacturing processes and relatively low unit costs—is the way to get there.
Q: What are some of the printing technologies that are unique to Thinfilm?
Cummings: We pioneer the use of printing to create integrated circuits—the hardware elements in the electronic devices that we commonly use.
The constraint you have in using silicon is that the number of devices you can build every year using chips—even though it seems enormous—actually scales with the number of people in the world, but not the number of ordinary objects. There are about 20 billion microprocesses manufactured every year (about three per person in the world). If we’re going to be adding electronics to consumer goods, then we have to go from billions of devices, to trillions of devices.
This leads to the uniqueness of Thinfilm. Using a different manufacturing technique, such as printing, can play a leading role. Printing doesn’t allow you to make the complexity of devices, like you can using silicon chips. The idea of intelligent agents on electronic devices requires a relatively sophisticated amount of computational power. But in our view, there is a tier of needs. You may have requirements for having a chip with that kind of power in relatively expensive devices, but you will also want simple information about the goods you buy. Are they authentic? Have they been previously opened or tampered with? What is the temperature they were stored at? What is their overall quality?
A memory sensor or detection system of some kind can help with each of these simpler questions that people ask regarding the products they intend to buy. And the amount of data you need to answer these questions is relatively trivial at times. It’s about small data, rather than big data. It’s about the ubiquity of having “smartness enough” in packaging or in devices to be able to answer those types of immediate questions.
Q: What do you hope to accomplish in your partnership with Ypsomed?
Cummings: Thinfilm’s NFC OpenSense™ tags, which are thin, flexible labels that can detect both the open or closed state of a package, will be applied to YpsoMate® autoinjectors, which are disposable 2-step injection systems that house pre-filled glass or polymer syringes. The solution will be called YpsoMate® Smart.
The tag will be applied on the autoinjector and the sensing loop will run over the cap. The patient taps the tag with smartphone before opening. A unique identifier that detects that the package is closed is sent to the cloud and doctor dashboard. The patient removes the cap, which breaks seal, and injects medication. Then taps the tag again and an opened ID is sent to cloud and to doctor.
Patients use autoinjectors for diabetes, growth hormones, fertility treatment, osteoporosis, autoimmune diseases (such as rheumatoid arthritis), and cardiovascular conditions.
At this point, the YpsoMate® Smart is a prototype and Ypsomed will drive the pilot testing and commercial roll out.
Q: How do you plan to accomplish these goals in your partnership with Ypsomed?
Cummings: We believe in a ubiquitous world where intelligence can be implemented in ordinary objects.
Our partnership with Ypsomed is all part of a multi-pronged approach to address several different markets with one technology that can solve many different challenges. There is a huge opportunity to bring this solution to other markets that require product authentication applications or industries, such as retail, that are looking for ways to better engage with customers and create physical-to-digital campaigns.
Q: How will these end goals impact the injectables market?
Cummings: Again, Ypsomed will be driving the pilot testing and commercial rollout. From a big-picture standpoint, this could mean the potential of receiving small data and other communication in a market where there are approximately 800 million disposable pen injection devices, and more than 70 million auto-injection devices (and that number is quickly growing). We’re looking for injectables to be smart, not just connected.
Q: How will these end goals impact pharmaceutical manufacturers, if at all?
Cummings: Pharmaceutical companies are focusing on biologically-produced preparations and, in particular, on monoclonal antibodies. Due to the size of the molecules, these have to be administered subcutaneously. That said, the injection is frequently only required on a weekly, twice-weekly, or even monthly basis. As great as this advantage is, it also poses a hidden (but serious) disadvantage—patients can easily get out of their injection routine or forget the next injection.
By leveraging small data that’s being delivered through smart labels, injectables have the potential to provide, in real time, the cost-effectiveness of treatments through a simple tap of a smartphone, thus have a clearer view of supply and demand.
Q: What do you see for the future of injectables?
Cummings: There will be more transparency when it comes to injectables. Doctors will be able to track whether or not patients are adhering to their self-injecting routines, while patients will have more accountability in terms of self-injecting. With access to real-time information, they will be kept aware of adherence history, injection reminders, usage guidelines, refill instructions, and authentication.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




