Stericycle Expert Solutions publishes quarterly Recall Index, revealing first quarter recall trends in the pharmaceutical space.
Every quarter, Stericycle Expert Solutions publishes a Recall Index, which breaks down recalls in various industries: consumer products, automotive, pharmaceuticals, medical device, and food and beverage.
Information is gathered for this report using “cumulative data from the four primary federal agencies that oversee recalls in the United States,” which are:
- The Consumer Product Safety Commission (CPSC)
- The Food and Drug Administration (FDA)
- The Food Safety and Inspection Service (FSIS) of the United States Department of Agriculture (USDA)
- The National Highway Traffic Safety Administration (NHTSA)
Michael Good, Vice President of Marketing and Sales Operations at Stericycle Expert Solutions, participated in an exclusive Q&A with Pharmaceutical Processing to go over some of the findings. His edited responses are below.
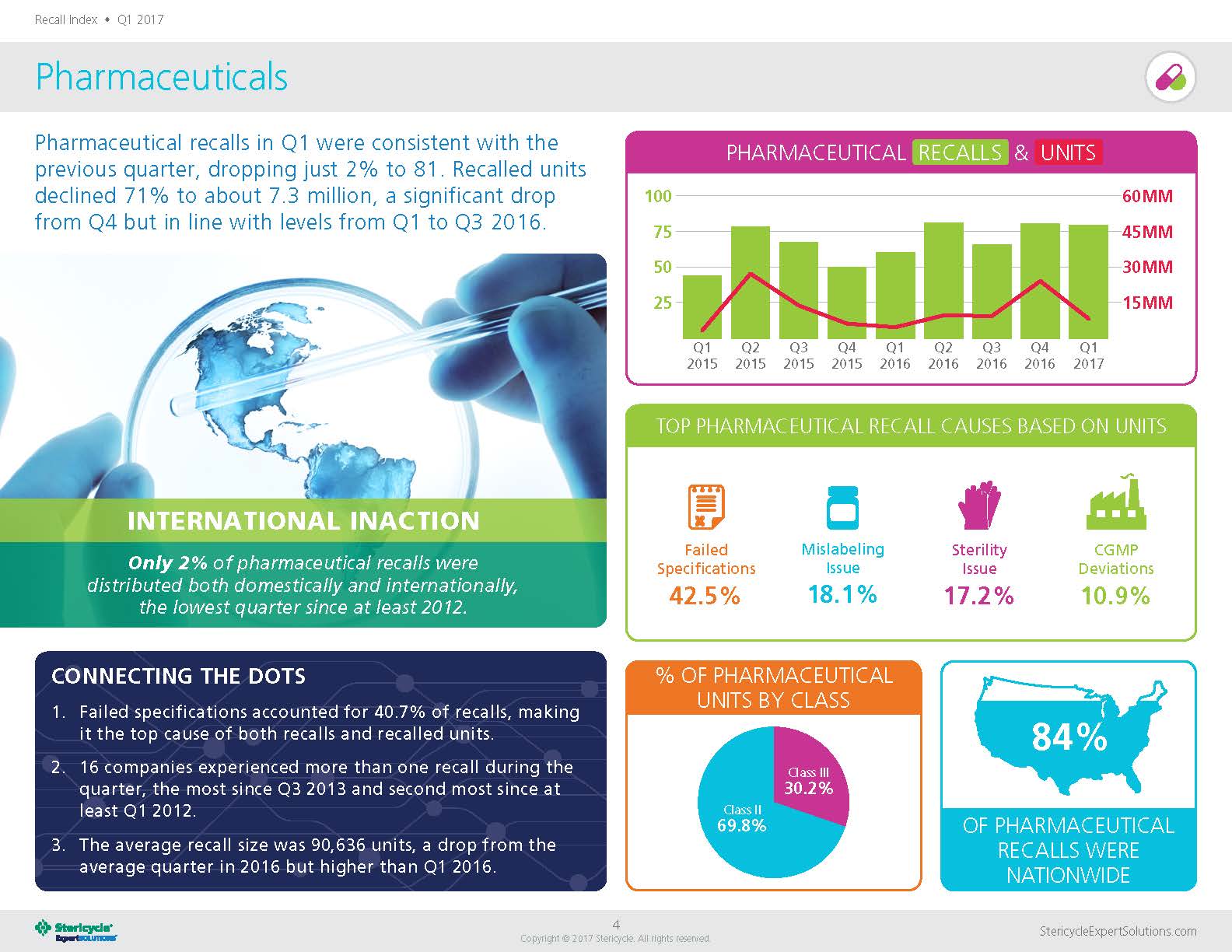
Image courtesy of: http://www.stericycleexpertsolutions.com
Q: In your Q1 2017 Recall Index, it says that pharmaceutical “[r]ecalled units declined 71% to about 7.3 million.” What impacted this decline (specifically since it was a significant drop from Q4 2016)?
Michael Good:
As is often the case, there wasn’t a single overarching factor that can explain the decline. However, Q4 2016 was higher than average—the third largest quarter since Q2 2012. So, in many ways, these findings represent more of a return to the norm rather than a significant plunge. It’s something we’ll be watching to see if there is a continued trend.
According to the Recall Index, “[o]nly 2% of pharmaceutical recalls were distributed both domestically and internationally” and 84% of pharmaceutical recalls were distributed nationwide. Why is such a high percentage of pharmaceutical recalls distributed domestically? Are there certain procedures or guidelines that are more difficult to comply with in the U.S., compared to internationally?
Good:
In the pharmaceutical industry, unlike, say, the food and beverage sector, there aren’t really any regional specialties or differences in cuisine. Pharmaceuticals are also governed primarily by the federal government, so there is no downside to distributing drugs nationwide. As for international distribution, any time there is more than one market involved, there are typically major differences in regulation that add to the complexity. In addition to the patchwork of regulations, there may be other challenges. For example, if multiple languages are spoken by consignees, there may be a need for contact center agents who speak various languages. But it’s also important to note that the number of recalls that fall into this particular metric doesn’t necessarily reflect how many pharmaceutical companies are operating in those countries.
Failed specifications accounted for 40.7% of pharmaceutical recalls. Could you dive into that a little further?
Good:
This issue consistently ranks at or near the top in terms of top causes of pharmaceutical recall activity. Any number of factors can lead to a failed specification situation. One common issue is stability testing, and since that measures how well the drug holds up over time, it is difficult to perform this test accurately before a product is distributed. So, it isn’t surprising that this is a common problem. It does show that pharmaceutical companies take quality testing seriously.
Sixteen companies experienced more than one recall during Q1. What were some of your findings there?
Good:
We don’t discuss specific companies, but the highest number of pharmaceutical recalls per company was four. By contrast, the medical device company with the highest number of recalls had 20.
The average recall size was 90,636 units. Do you foresee serialization and traceability implementation impacting the average recall size in the coming years?
Good:
Average recall size has already declined in recent years, and we believe serialization is one factor in this trend. In 2012, the average recall size was over 650,000 units per recall. Since then, the highest average annual recall size was roughly half that at 328,000 units per recall in 2014. Like other measurements, this figure fluctuates from year to year and quarter to quarter. In fact, in 2016, the average pharmaceutical recall increased throughout the year. But overall the trend line is down.
Of all of the causes for a recall of a pharmaceutical unit, what do you find the most concerning and why?
Good:
The biggest concern when it comes to pharmaceutical recalls isn’t necessarily the cause but rather the classification. Class I recalls present the most serious risk, but fortunately in the pharmaceutical space they are far less common than other recalls. Class II recalls, which the FDA defines as a situation that “may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote,” made up close to 70 percent of recalled units in Q1 compared to more than 84 percent in Q4.
Class II drugs had the highest number of recalls at 69.8%. Can you see any particular reason why Class II drugs, compared to drugs in other classes, had more recalls this quarter?
Good:
Historically, Class II situations have often been the highest percentage in the pharmaceutical space. There have been a few exceptions in recent quarters, when the less-hazardous Class III recalls led. Class I recalls are relatively rare, and that’s because the major causes that the FDA classifies as most serious, such as undeclared allergens and bacterial contamination, don’t occur often in the pharmaceutical industry. On the other hand, Class III recalls are also typically less common than Class II simply because of the nature of the product. Even a minor deviation in potency, for example, could potentially cause an adverse response, but it is likely to be temporary or reversible.
How can companies work to lessen the amount of pharmaceutical recalls going forward?
Good:
Companies should periodically review and update their preventive controls. This can help prevent certain recalls, such as mislabeling errors. But some issues, such as instability, are most likely to be discovered post-distribution. No matter what, recalls are going to happen. Along with focusing on recall prevention, companies should ensure their recall plans are as robust as possible and hold periodic mock recalls to test for various scenarios and identify any gaps in their procedures.
For more information, about the Recall Index, visit Stericycle Expert Solutions’ website.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




