
[Kadmy/Adobe Stock]
January 2023 saw a significant increase in the number of drug recalls and units recalled compared to the Q4 2022 monthly average.
1. Contamination: Leading cause of drug recalls in 2022 and early 2023
Contamination has consistently remained a central reason for recent drug recalls. In 2022 and early 2023, contamination issues continued to dominate the list of recall events.
Recent microbial contamination recalls have involved a variety of drug products, including oral suspensions, ointments and eye drops. Examples of microbial species involved in FDA recallinclude instances of Bacillus cereus, Burkholderia gladioli, Bacillus licheniformis, Bacillus sonorensis, Gluconacetobacter liquefaciens and Providencia rettgeri. This trend underscores the importance of maintaining proper sterility and quality control in drug manufacturing and storage.
Chemical contamination recalls have also been common in 2022 and early 2023. THe FDA has recalled products, for instance, after the detection of methanol in hand sanitizers. Other chemical contaminations include the presence of undeclared pharmaceutical ingredients like sildenafil and tadalafil in dietary supplements. The agency has also stepped up its enforcement of nitrosamine impurities and other potential contaminants in drugs in recent years.
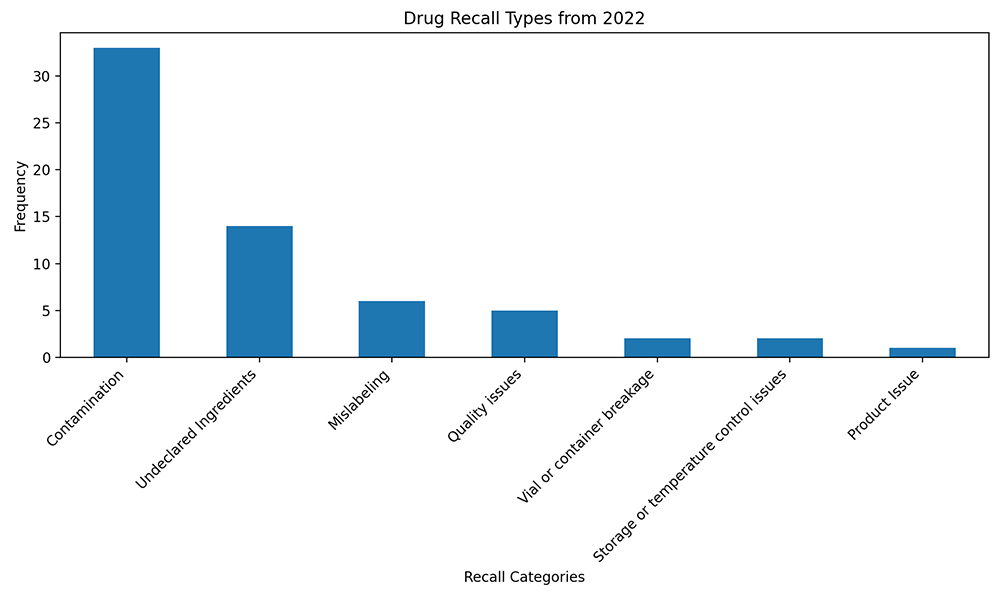
Drug recall types from 2022. Analysis of FDA recall data.
2. cGMP deviations and sterility concerns
Current Good Manufacturing Practices (cGMP) provide a framework for ensuring the quality, safety, and efficacy of pharmaceutical products. However, deviations from cGMP standards have emerged as a significant driver of drug recalls in recent times. In January 2023 alone, there were 13 recall events and 11.88 million impacted units as a result of cGMP deviations, according to research from Sedgwick. In 2022 and Q1 2023, sterility concerns were among the most common reasons for recalls. Compromised aseptic processes and product contamination were among the primary causes of these recalls, highlighting the need for robust quality control measures in pharmaceutical manufacturing.
Several pharmaceutical recalls in early 2023 highlight the ongoing challenges the industry faces in maintaining strict quality control and adherence to cGMP standards. Examples include recalls related to hand sanitizers from Midwest Cleaning Solutions, Clean Pro Supply, and Soft Hands as a result of the presence of methanol. In Addition, Ascend Laboratories recalled Dabigatran Etexilate Capsules because of the detection of the N-Nitroso-dabigatran (NDAB) impurity. Similarly, Purely Soothing and Apotex recalled their 15% MSM Drops and brimonidine tartrate ophthalmic solution, respectively, as a result of non-sterility or potential lack of sterility.
3. Failed specs another driver or quality control issues
Failed specifications accounted for a number of recalls in 2022 and early 2023. These recalls often resulted from inaccurate labeling or inconsistent potency, which can pose significant risks to patient safety. Examples include IBSA’s Tirosint-Sol (levothyroxine sodium) recall in February 2023 as a result of subpotency. In addition, Spectrum recalled epinephrine bulk API in January of 2023 as a result of product discoloration. Similarly, Accord pulled daptomycin for injection recall late last year as a result of mislabeling while Hospira recalled vancomycin injection around the same time as the result of the presence of visible glass particulates.
4. Undeclared ingredients: An ongoing cause of drug recalls
Undeclared ingredients in pharmaceutical products have been a persistent issue in recent years. Recently, several products have been recalled as a result of undeclared ingredients. For instance, in February 2023, PrimeZEN recalled Black 6000 male enhancement capsules because they contained undeclared tadalafil and sildenafil. In August 2022, Launch Sequence recalled Aphrodisia and Euphoria capsules as a result of undeclared tadalafil. Last July, FDA sent warning letters to four companies for illegally marketing honey-based products with unlisted active drug ingredients found in erectile dysfunction drugs. FDA noted that it confirmed the presence of contaminants in the products through laboratory testing.
5. Quality issues the cause of a significant number of recalls
Quality issues, while cited in five recalls in January 2023, impacted 1.32 million units, demonstrating the far-reaching consequences of lapses in quality control. Examples of recent quality issues leading to recalls involve mislabeling, vial breakage, label mix-ups, dissolution test failures and incorrect labeling. For instance, Accord Healthcare recalled Daptomycin for injection in late 2022 because of mislabeling. In November 2022, Exela Pharma Sciences recalled sodium bicarbonate Injection as a result of vial breakage. In September 2022, a label mix-up led Golden State Medical Supply to recall clopidogrel 75 mg and atenolol 25 mg tablets.





Informative article… thanks to the Author.
very scary i was using the eye drop liquid tears from india cost me a extra eye dr exam, and no way to get refund. fortunatly i had no problem dr has me on them after transplant for Fuchs’ dystrophy
i trying to search how many recalls are from foreign manufacture or ingredidents