Earlier this year, we took a look at the most common causes of drug-related recalls in 2022 and the first quarter of 2023. Now, we are expanding our lens to examine drug-related FDA recalls from 2011 to 2023.
Understanding the recall landscape
In recent decades, prominent incidents like the Tylenol poisonings in 1982 have underscored the need for attentive tracking, swift reaction and careful mitigation measures when facing possible hazards to health. To get a sense of the current landscape of pharmaceutical recalls, we analyzed an FDA dataset spanning from 2011 to 2023. The data, which includes an array of drugs and recall reasons, found that lack of assurance of sterility continues to be far and away the most common reason for pharmaceutical recalls.
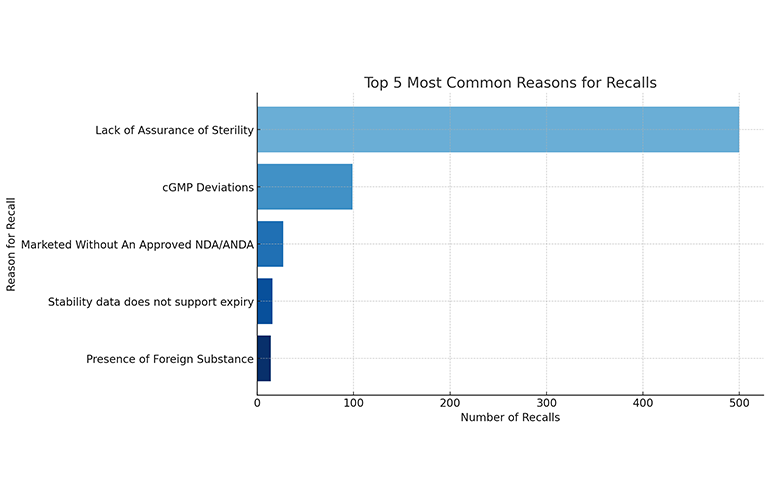
Sterility issues continue to clearly be the most common reason for FDA drug recalls. Data source: FDA
While FDA recalls of drugs have fluctuated widely over the past decade, there is a noticeable trend toward increased enforcement, particularly evident in 2022, which saw an unprecedented surge in recall volume. While recalls are trending lower so far in 2023, it is too early to tell if there will definitively be a drop over 2022 figures.
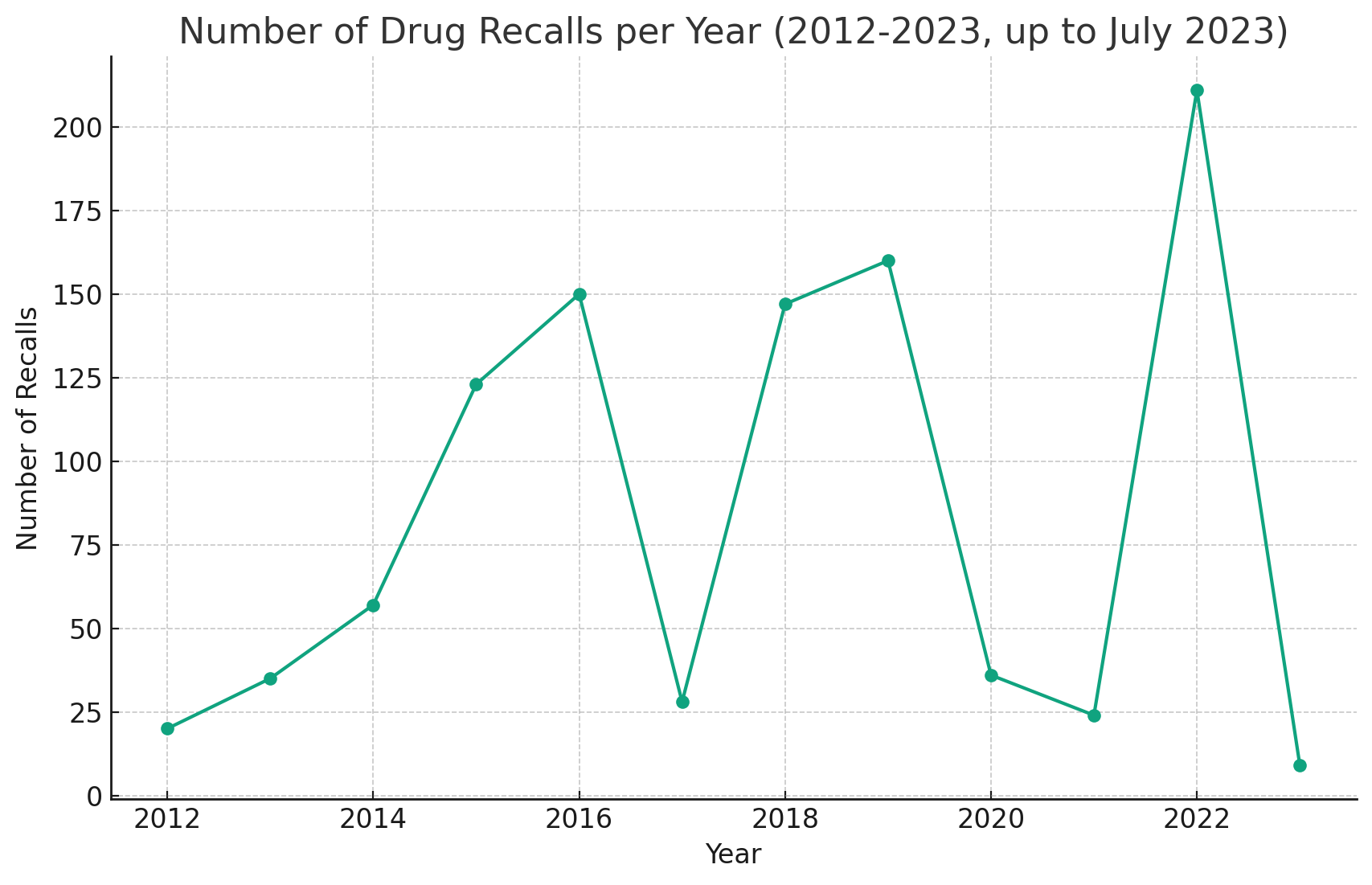
FDA recalls of drugs has fluctuated considerably over the past decade. Data source: FDA.
Spotlight on sterility challenges
Notable examples of sterility issues prompting FDA enforcement include a series of recalls of eye drops. Examples include the 2019 recalls of OCuSOFT eye wash sterile isotonic, tetcaine (tetracaine hydrochloride) ophthalmic solution, iSolutions ActivEyes nighttime lubricant eye ointment and Equate Support lubricating eye drops. The problem has persisted to the present with the recent recall of Global Pharma Healthcare eye drops.
cGMP deviations and unapproved drugs
Deviations from Current Good Manufacturing Practices (cGMP) were the second most common reason for FDA recalls. Examples of this recall issue include the recall of Westminster Pharmaceuticals’ irbesartan tablets, which are indicated to treat high blood pressure and diabetic kidney disease, in 2018 for cGMP violations. Similarly, Scrip Inc.’s 2020 recall of LidoPatch, a lidocaine topical analgesic patch, was the result of deviations in proper manufacturing protocols.
Rounding out the top three, selling pharmaceuticals without an approved New Drug Application (NDA) or Abbreviated New Drug Application (ANDA) was the next most common cause of recalls. Examples of this recall type include the 2017 recall of No. 1 Faiza beauty cream. More recently in 2021, an array of dietary supplements lacking proper NDA/ANDA approvals faced recall, including “Thumbs up 7 Red 70K” capsules, “Krazy Night” capsules and “Shogun-X” capsules. These recalls followed the discovery that the products contained the undeclared active pharmaceutical ingredients tadalafil, sildenafil and vardenafil. These ingredients are phosphodiesterase (PDE5) inhibitors found in FDA-approved erectile dysfunction treatments. Adulteration with such ingredients continues to be a common cause for recalls.
Insufficient stability data supporting expiry dates and the presence of foreign substances in products were also common causes of recalls. But these recall reasons were relatively rare compared to, in particular, sterility issues and cGMP deviations.
The role of technology and regulation
Although not a new technology, automation provides a proven strategy to help reduce pharma recalls as human error continues to be a common cause of problems that trigger enforcement actions. The FDA’s pledge to refine recall regulations, such as its guidance for voluntary recalls under 21 CFR Part 7, Subpart C, provides another layer of vigilance.
Yet the volume of recalls serves as a reminder of how common quality problems are in pharma. Over the past decade, the FDA has recalled more than 14,000 drugs, which works out to almost four per day, according to an article from Harvard Health Publishing.
Though such recall statistics underscore persistent challenges related to ensuring sterility and conforming to cGMP standards, they also demonstrate the industry’s capacity for evolution. The role of automation, which could be enhanced given the uptick in interest in AI, could enable manufacturers to continue integrating scientific advances while refining quality protocols.





Tell Us What You Think!