Despite a government-mandated, multi-year timeline for implementation, many industry experts are left questioning how to achieve and sustain unit-level traceability.
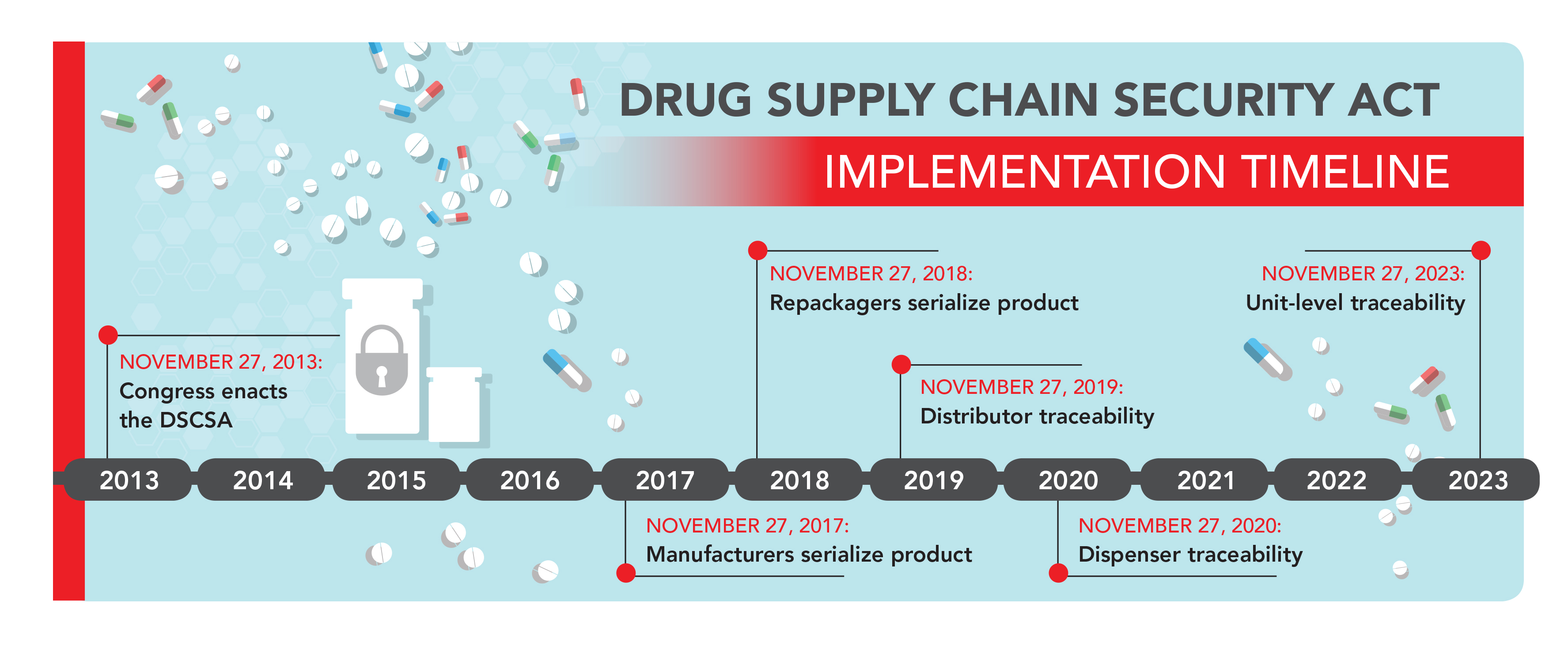
The Drug Supply Chain Security Act (DSCSA) was signed into law in November of 2013—enacted with the ultimate goal of better visibility across the U.S. pharmaceutical supply chain. Despite a government-mandated, multi-year timeline for implementation, many industry experts are left questioning how to achieve and sustain unit-level traceability.
Don Eberts, Senior Manager for Clarkston Consulting, participated in an exclusive interview with Pharmaceutical Processing on the DSCSA implementation steps and its inherent challenges. His edited responses are below.
In your opinion, what is one of the biggest misunderstandings surrounding the implementation of the DSCSA?
Eberts: Regarding unit level serialization, the law is clear. Aggregation is implied but not mandated by the law, which states that compliance efforts “…may include the use of aggregation and inference as necessary.” The omission of an unambiguous requirement for aggregation in the DSCSA has led some firms into asserting that aggregation need not be implemented before the November 27, 2023 deadline for full electronic tracking and traceability. This legalistic position may be theoretically correct, but it is practically meaningless, given the recent announcements by the Big 3 drug wholesalers, all of which are demanding aggregation as a condition of doing business, starting as early as 2018-2019. For serialization to be logistically feasible, aggregation will be needed for trading partner sales.

Along a similar line, what are companies’ biggest concerns in implementing DSCSA regulations, specifically serialization?
Eberts: The manufacturers’ initial concerns were centered on the effort to manage serialization equipment and software implementations. Most firms have made substantial progress in this respect. The next area of concern is how to transform serialization from a one-time implementation project into an ongoing business responsibility. Once implemented on the packaging unit, the exchange of data between trading partners at the serial number level is creating a new challenges based on the many relationships that manufacturers have with others in the supply chain. There are large varieties of manufacturers’ capabilities, with some totally outsourced to fully in-house packaging and information system capabilities.
As you know, the deadline for manufacturers to serialize products is November 27, 2017. Could you give an oversight of where the industry currently is?
Eberts: The majority of pharmaceutical manufacturers will be able to serialize products by the deadline; many will also be able to provide aggregation capability as well. Pharmaceutical wholesalers, particularly the “Big 3,” are making proactive efforts to prepare both manufacturers and themselves to meet the November, 27 2019 deadline. This is both for packaging labelling and for information exchange.
What are some of the big steps that manufacturers have to take in order to get to a place where their products are serialized?
Eberts: Manufacturers should, by now, be well advanced in selecting and implementing the systems required to generate, randomize, commission, and retain commissioned serial numbers; imprint or engrave serial numbers on unit level packages; and transmit Transaction History, Information, and Statements. Manufacturers will next need to concentrate on operationalizing serialization; that is, the transition of serialization from its current one-time implementation project status to a sustainable, on-going business and IT operating responsibility. Logistical challenges in the United States will require incorporation of new warehouse processes to record the serial numbers as part of the packing and shipping processes. This will be a significant effort. Exceptions in shipping will require coordination of physical processes with information systems transmissions.
What do you foresee as the greatest challenge for manufacturers to meet the 2017 serialization deadline?
Eberts: Manufacturers need to sustain their efforts to install and test systems and create or revise procedures to ensure that their plants and CMOs will be ready to produce serialized products by the 2017 deadline. In particular, they need to ensure that they order equipment as early as possible and closely manage their project in conjunction with their equipment and system vendors. The limited capacities of these vendors, combined with escalating demand for their services, is resulting in rapid increases in both lead times and costs. Some system vendors have been refusing new business, while others have been rapidly expanding their workforces. But their new personnel inevitably do not yet possess the skills and knowledge of their more senior colleagues. Internally, manufacturers need to concentrate on operationalizing their serialization efforts in order to transfer serialization knowledge from the members of their implementation project teams to their ongoing business and IT operations personnel. This requires change management assessment and impact analyses, both on personnel and on budgets, to ensure that they will be able to incorporate serialization without disruption as part of their normal operations starting from the deadline. These efforts should now be underway.

Do you see any difficulties in manufacturers meeting this deadline as an industry?
Eberts: The steps remaining to ensure compliance and the ability to operate under the forthcoming serialization requirements are clear, as noted above. Any difficulties would stem from inadequate planning or inability to obtain sufficient external and internal resources to execute those steps. The FDA granted a six-month grace period to manufacturers to meet the lot-level traceability requirement that had been scheduled to go into effect on January 01, 2015. It would, however, be reckless behavior on the part of manufacturers to assume that the FDA might grant a similar grace period for the November 27, 2017 deadline. After all, manufacturers will have known of this deadline for four years by then, certainly sufficient time for them to prepare to meet it.
What are some of the big hurdles that repackagers will need to overcome in order to meet the November 27, 2018 deadline and be in compliance?
Eberts: The project organization, equipment, and systems that repackagers will need to implement closely resemble those of manufacturers. Given the demand on the vendors of those equipment and services by manufacturers, coupled with the increases in vendors’ equipment lead times and demand on their personnel, repackagers would be wise to start their compliance efforts sooner rather than later. Similarly, implementing information systems to record serial numbers will also be a challenge.
What are some of the key steps for repackagers to be prepared to serialize their products?
Eberts: Like the manufacturers before them, repackagers will need to:
- Define the scopes of their compliance efforts
- Establish budgets
- Assign personnel
- Establish project management organizations
- Obtain external resources, if necessary
- Select serial number management, printing/laser engraving, and networking systems
- Create or revise procedures
- Train their operations personnel to manage serialization on an on-going basis

Distributors have a traceability implementation deadline of 2019. Let’s give a quick recap first—where is the industry currently? What are some of the next steps for distributors?
Eberts: The Big 3 drug wholesalers are essentially united in their common position of requiring aggregation from manufacturers. In their judgment, aggregation and inference are essential to effectively manage their businesses, once serialization comes into effect.
Two trends appear clear:
- Other wholesalers and specialty pharmaceutical companies will follow the Big 3 lead and also require manufacturers to aggregate their products well prior to the wholesalers’ November 27, 2019 deadline.
- Wholesalers will almost certainly levy handling fees on any manufacturers who fail to aggregate their products.
What do you foresee as one of the biggest challenges for all segments of the industry to have implemented?
Eberts: Aggregation. Unit level serialization is straightforward. Its requirements include central or regional serialization repository systems (L4), line equipment and software (L2) and, in some cases, plant level control systems (L3) and, in some cases, line and/or building alterations, combined with the project organizations and budgets required to implement them in time to meet the deadline. Aggregation compounds the manufacturers’ implementation challenges, as it requires significant increases in equipment and may result in additional impacts on line and building layouts, thereby impacting implementation schedules and budgets. Manufacturers that have not been planning all along to include aggregation in their plans may be able to meet the unit-level serialization DSCSA deadline but risk facing additional costs and production disruptions to meet the aggregation requirements effectively being imposed on them by the major wholesalers.
Going forward, there’s going to need to be a lot of data sharing once all of these regulations are implemented. What do you see as some of the biggest challenges here?
Eberts: Failure to take advantage of opportunities this unprecedented amount and quality of data will provide. All supply chain parties, from manufacturers through dispensers, would be wise to assess not only how to meet the Transaction History, Information, and Statement data requirements mandated by the DSCSA, but how they could use these data to their business advantage.
Would you say there’s a different level of difficulty for various companies along the supply chain to be in compliance?
Eberts: Manufacturers’ packaging line equipment and systems investments will be more extensive than those of their downstream supply chain partners, but all parties have to invest in serial number repository/control systems, scanning equipment and systems, and the interfaces among these systems. Additionally, all parties have to invest the time and effort to train their operations personnel and establish or revise their operating procedures to incorporate serialization responsibilities.
In your opinion, what will be the greatest benefit from the DSCSA once it is fully implemented in 2023?
Eberts: Patients should ultimately benefit since the law will make it easier to identify and remove counterfeit pharmaceutical products from the supply chain, assuming that manufacturers, wholesalers, repackagers, and dispensers live up to their responsibilities as defined by the DSCSA. Pharmaceutical manufacturers, in particular, stand to gain significant business benefits from their investments in serialization. Among the potential benefits are having the data in sufficient detail to improve supply chain management, support sales and marketing campaigns, monitor and optimize performance by plants and CMOs, improve cold chain management, assess product availability with respect to markets, minimize losses resulting from product theft or diversions, and the ability to issue returns credits at original instead of (usually higher) current prices. Downstream supply chain partners will also have the potential to realize some of these business benefits from their serialization investments.
This article can also be found in the July 2016 issue of Pharmaceutical Processing.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




