Process design, measurement, and control for enabling continuous processing adoption in pharmaceutical manufacturing.
The shift from batch to continuous production methods is transforming the future of pharmaceutical manufacturing. The potential economic gains from increasing capacity utilization and reducing the length of process development, product release times, and capital costs are driving the paradigm shift. Spurred by potential improvements to quality, patient safety, and the time required for breakthrough medicines to reach patients, even the U.S. Food and Drug Administration (FDA) has advocated a move to continuous manufacturing (CM).1 However, with new methods come new challenges—particularly for process design, measurement, material traceability, and control.
Manufacturers, suppliers, and research institutions are collaborating to solve these challenges at projects across the globe, including the Engineering Research Center for Structured Organic Particulate Systems (C-SOPS), based at Rutgers, the State University of New Jersey. This article provides a summary of the status of continuous processing in the pharmaceutical industry and considerations—based in part on research at C-SOPS—for how manufacturers can overcome the challenges related to quality, compliance, material traceability, and process design and control.
Drivers for Continuous Manufacturing
The economic benefits of continuous methods over batch exist in nearly all areas of manufacturing and are the primary reason most industries have used continuous methods for decades. The FDA and other agencies have recently made it clear that they will not only accept CM, but that they support it as an enabler for true Quality by Design (QbD).
In the case of secondary solid dosage, CM allows for much faster QbD-driven process development and requires less raw material. This means that pharma companies can bring products to the market faster, with better quality and at a lower cost, adding up to significant savings and potentially billions if first to market is a factor.
Financial advantages exist even if the product is not a blockbuster. Continuous facilities will have less in-process material that ties up capital and they will operate at a much higher capacity compared to batch facilities, enabling CM to deliver production requirements with smaller equipment, smaller building footprints, and less capital investment.
New Implications for Quality and Compliance
Continuous manufacturing presents new questions for how to ensure quality and regulatory compliance. The primary question is how to maintain consistent process control and quality when there is no break point to hold back production from one unit to the other. The quick answer is to monitor critical process parameters (CPP) and critical quality attributes (CQA) at a frequency that allows for sufficient monitoring of the process and product quality. In addition, the real-time application of Process Analytical Technology (PAT) for continuous processes is essential to quality control and must be integrated into the process control strategies.

In a typical continuous process for tablet production, PAT instruments are important to monitor for homogeneity of the output of a continuous blender, moisture of the output of a fluid bed dryer, particle size of the output of the granulator, percent API of material going into the tablet press, and weight and hardness of the final tablets. The use of validated process models that execute in real-time with the process can predict these and other CQAs.
Practitioners of CM will need to have a much higher level of process understanding and more advanced quality measurements and controls, but the result will be a significant increase in overall quality. The advancement of CM might thus lead to an increased expectation for quality within the industry and regulatory agencies. In the future, this could make it more difficult for batch processes to get qualified.
Maintaining Material Traceability
Another facet of the quality and compliance question is how to trace incoming raw and intermediate materials to the final product when using a process that lacks a natural, discrete separation of materials inherent in batch. Material traceability is especially important in the pharmaceutical industry where manufactured goods are scrutinized for regulatory compliance, safety, and efficacy.
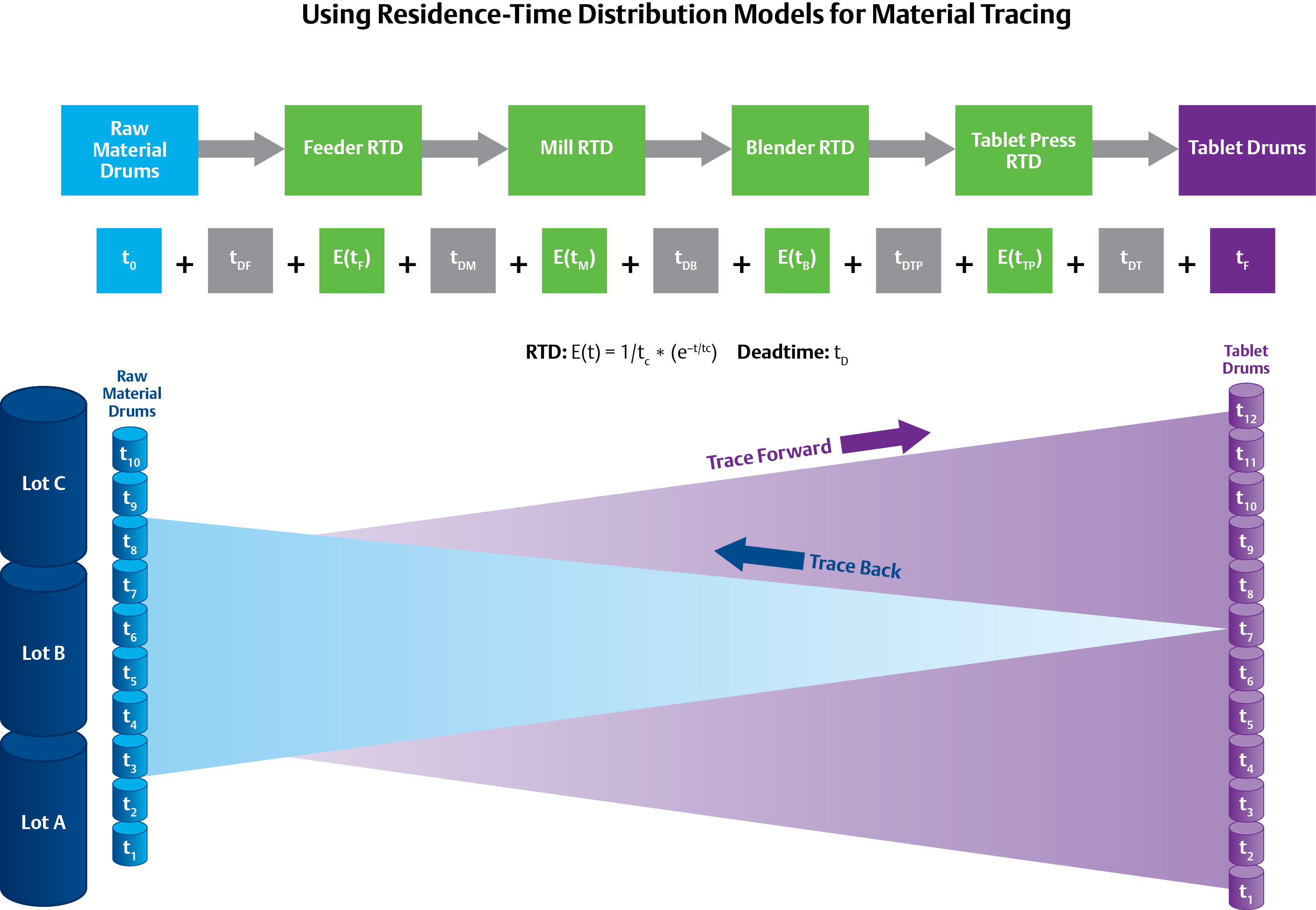
The use of Residence-Time Distribution (RTD) models, as seen above for the DeltaV™ distributed control system, can help trace materials backwards and forwards, but relies on statistical probability rather than physically discrete batches of material.
The traceability requirement can be complex in continuous processes because it relies on statistical probabilities rather than physically-discrete batches of material. It may only be possible to predict, for example, that there is a 90 percent chance that the material in a particular tablet came from Lot #1, a 9 percent chance that it came for Lot #2, and a 0.9 percent chance it came from Lot #3. The probability distribution depends on a concept called Residence Time Distribution (RTD)—a function that describes the amount of time a particle could spend inside the vessel.
C-SOPS has developed models for unit operations to predict the RTDs under different process situations. The utilization of these RTD models can provide significant savings by maximizing production. In the case of transient off-spec production, the accuracy of the models could mean the difference between disposing of a minute’s worth of production versus a few days.
The RTD of a unit operation such as a feeder, blender, or tablet press can be determined by a trace or step test. This data is combined with first principle models to accurately predict the states of the individual unit operations. The use of these types of models versus pure empirical methods has the advantage of being able to predict RTDs dynamically based on process parameters including flow rate, mixing speeds, and density. If a PAT application using an NIR analyzer to predict a CPP after the blender is out of specification, the RTD models can be used to predict when and which tablets need to be rejected. These RTD models can be integrated into a process control system to provide synchronized real-time material traceability and process control.
The Right Mix of Process Control
We only have to look at other industries to see how the pervasive use of process control systems and advanced controls can maximize quality, productivity, and profitability. In addition, the FDA wants assurance that if there is an upset in the process, the system is robust enough to maintain product integrity and reject off-spec production, which will require some level of real-time measurement, analysis, and control. The benefit is the potential for decreased regulatory inspections and audits.2
The idea behind advanced controls and optimization is to control in a much narrower and desirable range of the design space than is mandated. By controlling within a narrower range, the process will be more robust and typically result in higher quality and throughput.
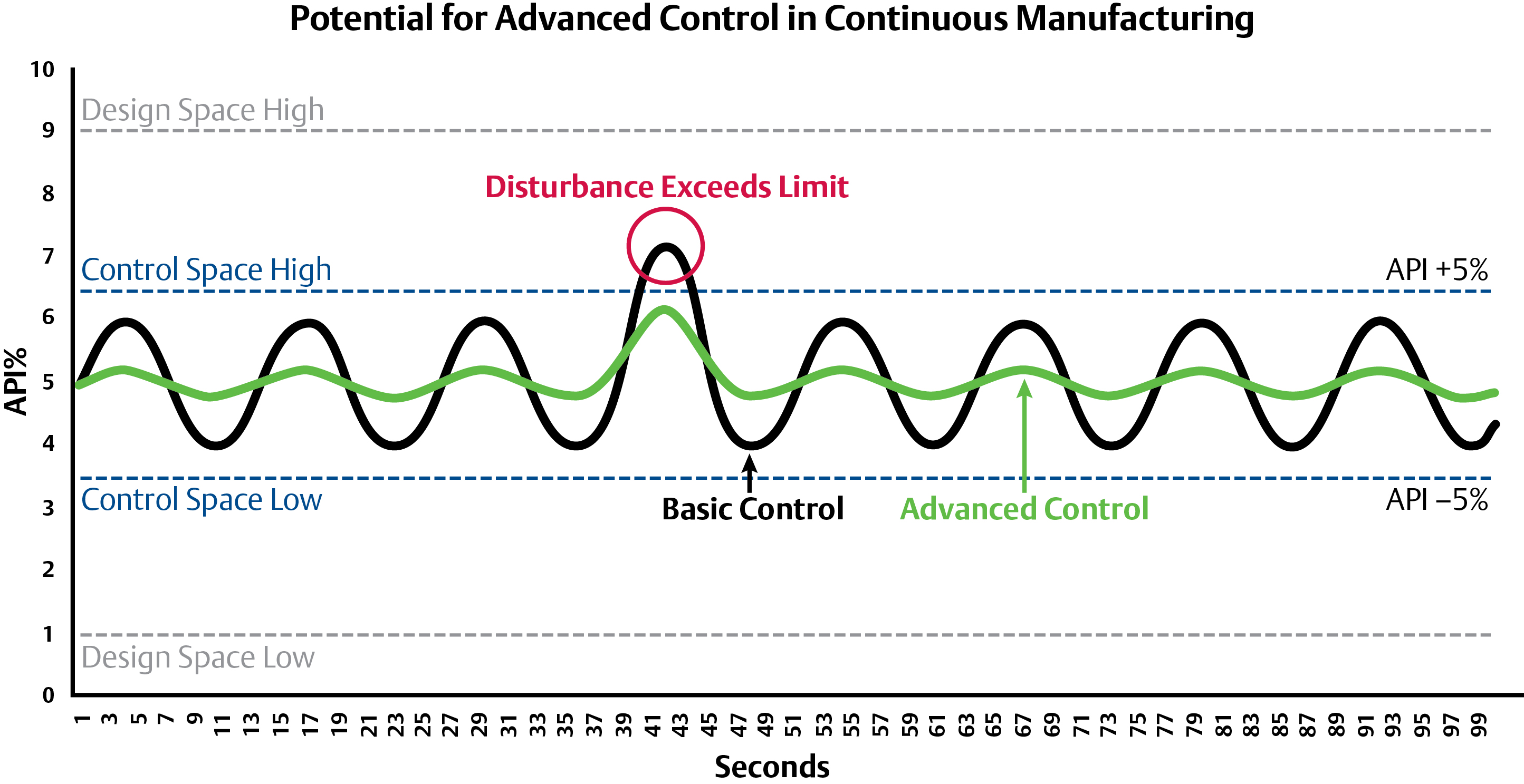
Advanced Controls have the potential to reduce process disturbances and avoid process off-spec material. The controls have reduced variability to within +/-1% in other industries and similar results may be possible in pharma.
Advanced controls have been implemented and tested at C-SOPS for both feedforward and feedback. In the case of feedback control an NIR analyzer was placed at the discharge of the continuous blender to measure the API concentration. A Model Predictive Controller (MPC) was used in closed-loop control to maintain the API concentration by varying the ratios to the feeders. Experiments showed that the MPC can minimize a disturbance from the feeders and bring the system back to a steady state faster, thus minimizing the potential for off-spec production and subsequent rejection. C-SOPS is also performing tests using this same NIR analyzer to feedforward compression and fill-depth settings to the tablet press to reduce variability in tablet hardness, weight, and density which directly affect tablet dissolution properties and the efficacy of the drug.
In both cases, the feedforward and feedback loops are a form of optimization. Even if producers can decrease the quality of the API concentration in a tablet from +/- 5-10 percent to 2.5-7.5 percent, there is still a lot of waste compared to improving product variability down to +/- 1 percent—which is typical in many industries.
In addition, the RTD models can also be used to optimize the plant design and to optimize production unit operations. These models can execute in real-time and be integrated with the process control system and PAT instruments. Emerson, QbD Process Technologies, and C-SOPS have developed a working system that integrates these components into a process control system for continuous solid dose manufacturing.
C-SOPS also has conducted materials characterization tests for density, cohesion, wettability, friability, flowability, electrostatics, bridging, and dissolution. The results of these tests form the basic material characteristics of the product or formula. These characteristics can be tested with the models to assess how these materials will perform in continuous processes and to identify changes in the formula that can result in better performance.

Uncovering New Opportunities Through Collaboration
As the paradigm shifts from batch to continuous manufacturing over the next decade, the advantages of advanced process control within continuous processing will become clear as manufacturers look to push the efficiency envelope while simultaneously improving quality and reducing the time to market. Highly successful public-private partnerships like C-SOPS have fostered the development and demonstration of these and other enabling attributes of continuous pharmaceutical manufacturing. Emerson and QbD Process Technologies, working with its partners and clients in these partnerships, is leading the way for designing the optimal control system platform that integrates the concept of QbD with PAT, advanced control, and material traceability for a particular product or process.
References
- Brennan, Zachary. “FDA calls on manufacturers to begin switch from batch to continuous production.” www.In-Pharmatechnologist.com. 1 May 2015.
- Woodcock, Janet. “Modernizing Pharmaceutical Manufacturing – Continuous Manufacturing as a Key Enabler.” MIT-CMAC International Symposium on Continuous Manufacturing of Pharmaceuticals, 20, May 2014.
About the Authors:
Doug Hausner, Associate Director for Industrial Relations with Engineering Research Center for Structured Organic Particulate Systems (C-SOPS), Rutgers University.
Jonathan Lustri, Senior Life Sciences Consultant for Emerson Process Management with over 30 years of industry experience in process development, R&D, product development, and process consulting.
Paul Brodbeck, Chief Technologist QbD Process Technologies, Inc. and a mentor for the Engineering Research Center for Structured Organic Particulate Systems (C-SOPS), Rutgers University.
This article can also be found in the March 2016 edition.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




