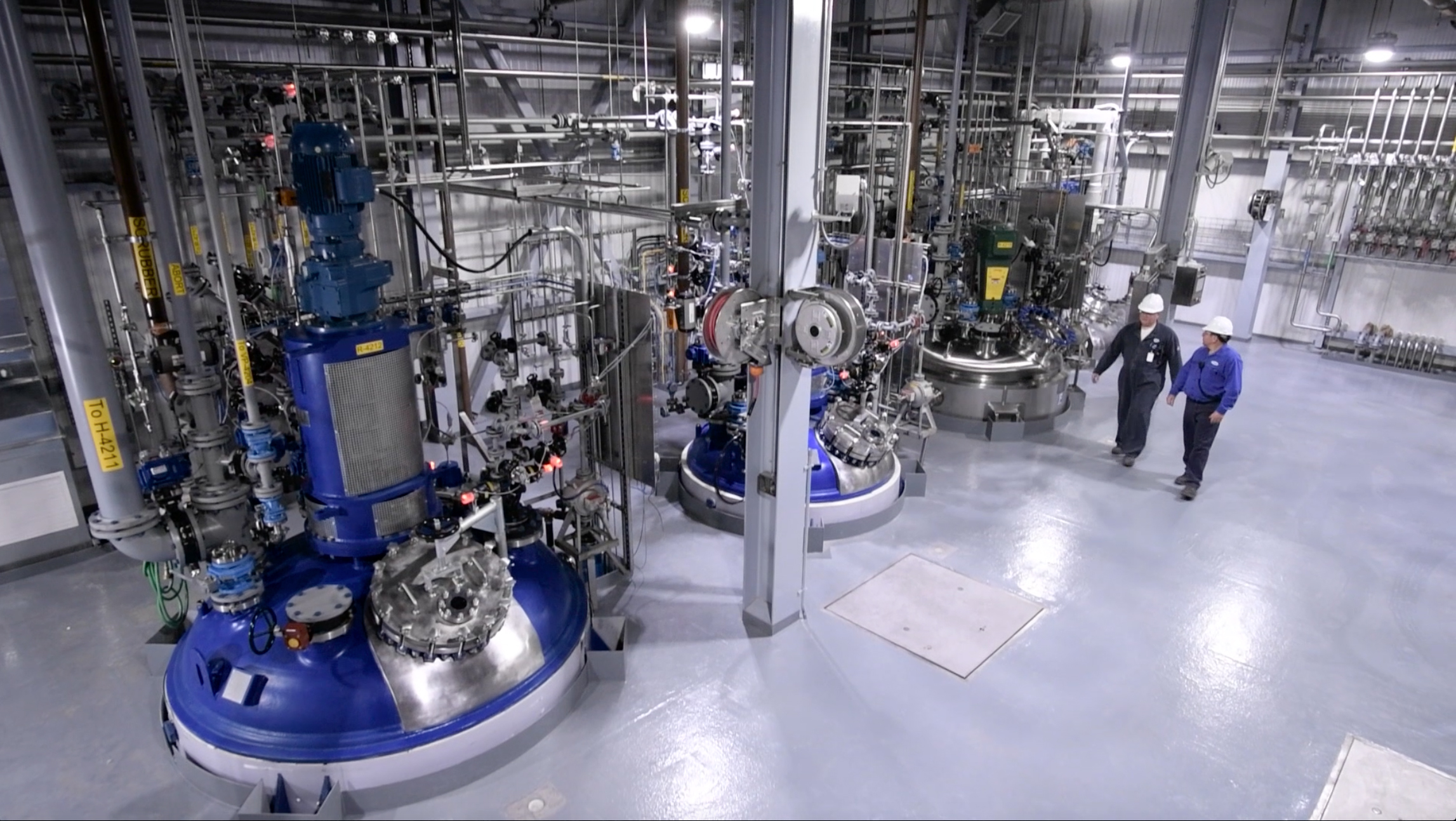
Operations at the Cambrex Charles City, IA plant. (Credit: Cambrex Corporation)
Cambrex Corporation, a manufacturer of small molecule innovator and generic Active Pharmaceutical Ingredients (APIs), announced Tuesday an investment to expand chemical and analytical development capabilities at its Charles City, IA plant. The project forms part of the company’s strategic plan to increase its development capacity and resources in North America.
The expansion will see the construction of an additional 2,000 sq. ft. of laboratory space for development projects, and will support the hiring of an additional 14 chemists. The building, installation and validation of equipment is expected to be completed by 1Q 2018.
The expansion at Charles City is in parallel to the investments announced in 2017 at Cambrex’s facility in High Point, NC where a new 11,000 sq. ft. analytical laboratory and 400 sq. ft. pilot plant with a total reactor capacity of 4,000 liters are currently being completed. These expansions at High Point will create up to 18 new jobs.
“The Charles City site has seen multiple investments and expansions in manufacturing capacity over the past five years to meet market demand, and this in turn, has led to us needing to increase the capacity of the supporting development and analytical functions,” commented Joe Nettleton, vice president U.S. operations, Cambrex. “Our strategy in expanding the development capabilities across the sites at both Charles City and High Point is to ensure both facilities are harmonized and can function synergistically so that projects can be seamlessly transferred between the two.”
Cambrex’s Charles City, Iowa facility is located on a 45-acre site and manufactures a wide range of APIs and pharmaceutical intermediates, including highly potent molecules and controlled substances. The High Point site, formerly PharmaCore, Inc., was acquired by Cambrex in October 2016, and produces complex APIs and intermediates in batch sizes from milligrams to 100kg to support clinical trials from Phase I to Phase III.
(Source: Cambrex Corporation)




