Abeona Therapeutics receives orphan drug designation in the European Union for EB-101 gene therapy clinical trial for epidermolysis bullosa.
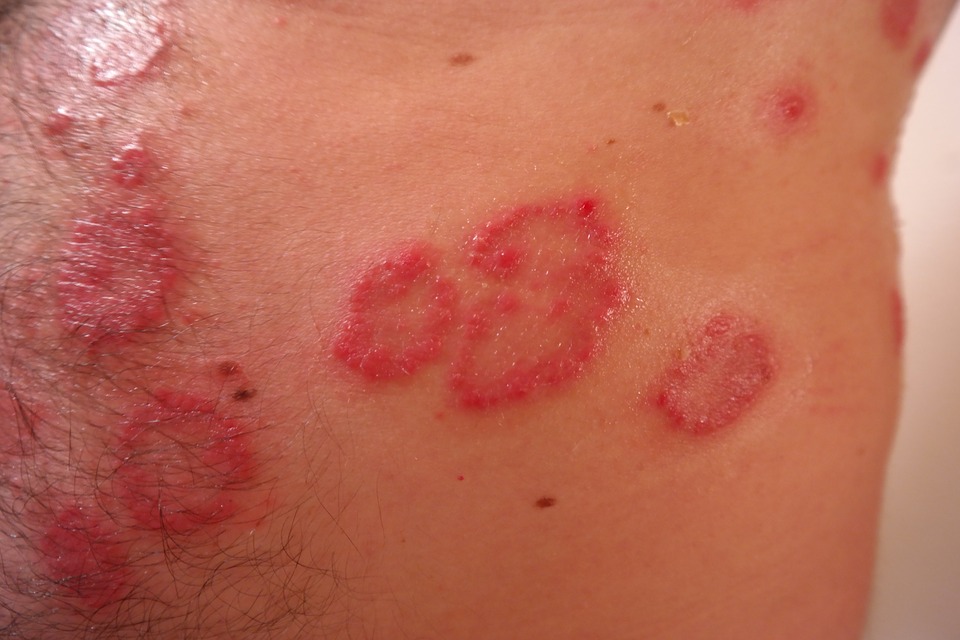
Abeona Therapeutics Inc., a clinical-stage biopharmaceutical company focused on developing gene therapies for life-threatening rare diseases, announced today that the European Medicines Agency (EMA) Committee for Orphan Medicinal Products has granted Orphan Drug Designation for Abeona’s EB-101 gene therapy program for patients with recessive dystrophic epidermolysis bullosa (RDEB), a devastating, life-threatening genetic skin disorder that is characterized by skin blisters and erosions all over the body.
“EB-101 is Abeona’s fourth gene therapy program to be granted EMA Orphan Designation and it further builds on our commercial portfolio of clinical-stage gene therapies that have received FDA and EMA orphan drug designations, which is an important validation of the clinical translation of these treatments for severely underserved patient populations,” stated Timothy J. Miller, Ph.D., President & CEO of Abeona Therapeutics Inc. “The orphan designation also provides 10 years of market exclusivity in the European Union from similar medicines for similar indications, an important value driver for Abeona as we expand our clinical programs in Europe.”
The ongoing phase 1/2 clinical trial with gene-corrected skin grafts has shown promising wound healing and safety in patients with RDEB. Investigators at Stanford University are enrolling adolescent and adult patients for the phase 2 portion of the EB-101 clinical trial to determine the efficacy of COL7A1 gene-corrected grafts on wound healing (NCT01263379).
Typically, wounds in patients with RDEB, also known as “butterfly skin” syndrome, can remain unhealed for months to years due to the inability of the skin to stay attached to the underlying dermis and can cover a large percentage of the body. Results from the initial four patients of the clinical study demonstrated that treatment with EB-101 restored type VII collagen (C7) expression at the dermal-epidermal junction at the graft sites in 90 percent of the biopsy samples at 3 months post-treatment, in 66 percent at 6 months post-treatment, and in 42 percent samples at 12 months post-treatment.
Importantly, correct type VII collagen localization was observed at anchoring fibrils. Wounds that demonstrated type VII collagen at graft sites displayed 87 percent healing at 3 months, 67percent at 6 months, and 50 percent at 12 months compared with baseline wound sites.
“The encouraging EB-101 clinical results advances our support to address the significant unmet medical needs that RDEB patients experience and underscores our commitment to collaborating with outstanding research groups such as Stanford University,” noted Steven H. Rouhandeh, Executive Chairman of Abeona Therapeutics. “We are also very proud to collaborate with dedicated patient advocacy groups such as EB Research Partnership (EBRP) and EB Medical Research Foundation (EBMRF) to advance EB-101 for the RDEB community.”
(Source: GlobeNewswire)




