Counterfeit drugs have become an increasing global threat over the last few decades. More and more regulatory authorities, including those of Argentina, Brazil, India, South Korea, Saudi Arabia, and Turkey, have adopted or will adopt requirements for the use of globally unique drug identifiers and serialization in order to protect the supply chain. These new rules, of course, are in addition to existing and pending track-and-trace regulations in the U.S. and the EU.
The U.S. Drug Quality and Security Act (DQSA)
The Drug Quality and Security Act (DQSA) was signed into law in 2013. It outlines critical steps to build an electronic, interoperable system for identification and traceability of prescription drugs as they are distributed in the U.S. The new law supersedes any individual state guidelines. The requirements are phased in over a period of 10 years by providing a migratory path from lot traceability to serialization to item-level traceability. The next big step will be the introduction of mandatory medicinal product serialization in 2017.
EU Falsified Medicines Directive
One of the key objectives of the European Directive on preventing falsified medicinal products from entering into the supply chain is to ensure product integrity and authentication of medicines, i.e. safety features and product serialization. To effectively accomplish this means a harmonized unique identifier across Europe. Adopted in 2016, the Delegated Acts on safety features (Regulation 2016/161) lay out the technical parameter of the greater EU Falsified Medicines Directive (FMD), setting up the first “end-to-end verification system” for drugs across the world. The requirements must be implemented by EU member states by February 2019 (2025 for Belgium, Italy, and Greece). However, implementation at national level can be completed before the end of these transition periods.
Solution: End-to-End Verification System
Prerequisite for the protection against falsified medicines is the unique assignment of a serial number in conjunction with individual production data as data matrix code (product identification GTIN/NTIN/PPN, expiration date, and batch number). Together with seal labels at the tops and bottoms of folding boxes for physical protection against manipulations, the unique serial number system ensures the authenticity and integrity of medical products.
Millions of serial numbers—generated by pharmaceutical companies or provided by authorities—have to be organized, printed, and sent to national verification processes. Finally, the serial numbers of all prescription drugs will be scanned at pharmacies for verification against a central database. Only if the serial number is valid, and the medical product thus authenticated, will it be handed over to the consumer.
By 2020, experts expect approximately 90 percent of prescribed medications worldwide to bear such a serial number. That leaves less than four years to make up a very sizable gap.
Track-and-Trace Solution Requirements
To fulfill these regulatory requirements for process workflow, pharmaceutical and biopharmaceutical companies need a reliable track-and-trace solution, which should provide the following features:
- Compliance with international anti-counterfeiting requirements
- Support of GS1 labeling information
- Management and randomization of serialization numbers in operations
- Management of modular aggregation for item, bundle, pallets
- Integration with centralized track-and-trace repositories using standards like EPCIS
- Handling of packaging orders and batch information
- Dialogs to create, discard, aggregate, and disaggregate units and hierarchies
- User management, including rights management & audit trail
- Recording and reconciliation of used and unused serialization numbers

Editorial Credit – Akimov Igor Shutterstock.com
System Architecture According to ANSI/ISA S95
According to the internationally accepted ANSI/ISA S95 standard, an efficient track-and-trace solution should integrate level 4 (see chart below) with the packaging equipment and line controllers on the shop floor (level 2) via standard interfaces.
Level 4 comprises the Enterprise Resource Planning (ERP) for the administration of material master data, as well as the Central Repository typically responsible for generating and randomizing serial numbers. The track-and-trace solution must be able to submit these numbers to different line systems at level 2. (Please note that, in some scenarios, no level 4 repository may be required; in such situations, the level 3 track-and-trace solution should also be able to generate serial numbers and act as a long-term archive for these numbers, at least at site level.) Afterward, the used serial numbers—including their status—must be returned and stored in the Central Repository.
In the case of contract manufacturing organizations (CMOs), the pharmaceutical company customers (Marketing Authorization Holder, or MAH) act similar to the central repository, providing serial numbers for the CMO, who in turn reports back to the MAH.
This architecture must ensure seamless information exchange in both directions and must be flexible enough to catch errors before unacceptable product and time loss accrue. For example, production should be able to be immediately stopped in instances of missing or inconsistent data to avoid manufacturing unusable (i.e. incorrectly serialized) products.
Benefits of Track-and-Trace and Manufacturing Execution System (MES) Integration
The track-and-trace solution can also be directly integrated into a Manufacturing Execution System (MES) on level 3. The benefits:
- Seamless integration of Electronic Batch Records (EBR) and serialization order
- Batch Record Reports (BRR) include serialization data
- Only one graphical user interface for the operator
- Fully automated stock creation
- Central maintenance of material master data
The ERP system links business process areas such as procurement, resource planning, sales, human resources, and finance by means of a common data basis. The central number management system providing the serial numbers also is located on this same level 4. Imprinting individual packages with serialization numbers is one sub-process on level 2.
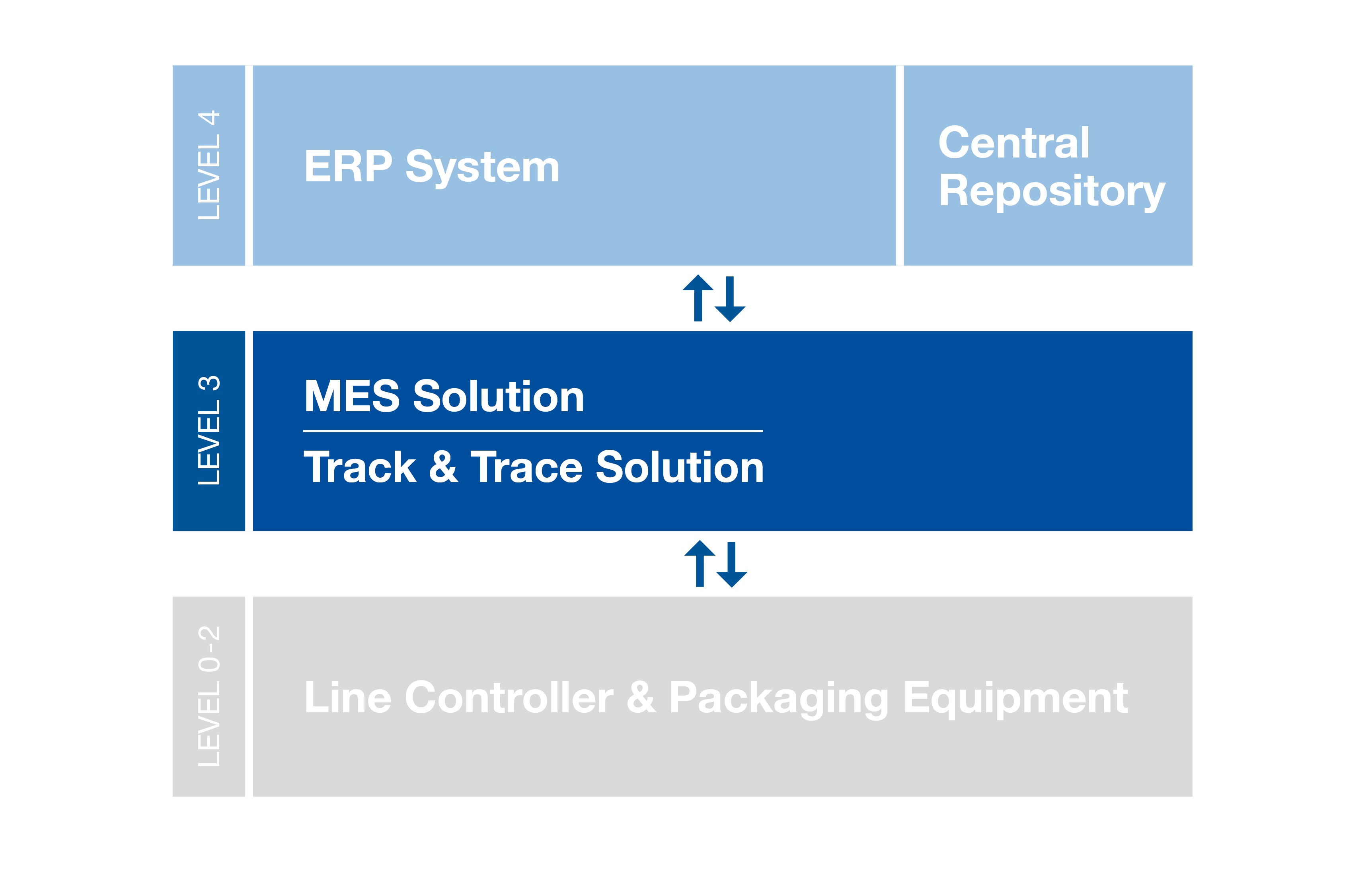
In this organizational setup, the MES at level 3 integrates the ERP and the Central Repository with the shop floor packaging equipment and line controllers in turnkey fashion.
Implementation Challenges for Track-and-Trace Solutions
The implementation of track-and-trace projects will be a great technical and personnel challenge for pharmaceutical and biopharmaceutical companies, as it will touch all departments. Due to missing standards, serialization solutions must be adapted to existing processes in the internal value chain according to the unique and often disparate project execution methods at various companies around the world. In turn, suppliers must integrate requirements such as coding, inspection, integration, and data management into existing processes.
In addition, pharmaceutical manufacturers are understandably concerned about generating higher one-off expenditures, as well as incurring costs caused by production outage, additional equipment, and, per the high complexity of fully integrated information systems, inevitable upgrades to both software and hardware. It can take approximately two years from initial planning—process analysis, supplier searches, etc.—through to the implementation and verification of functional track-and-trace solution and a staff trained in its proper utilization.
Standardization of Data Exchange: Open-SCS Working Group
To accelerate project execution, the development of an open standard is of significant importance. This standard should cover data exchange during packaging serialization for the entire business transaction. This is one of the main targets of the Open-SCS Working Group, a working group hosted by the OPC Foundation to which our company, Werum IT Solutions, also is committed. Open-SCS will define global serialization use cases across all levels, including interfaces between levels 2 & 3 and 3 & 4 for level 2 plant (line and equipment) and supply chain packaging serialization activities (distribution centers and warehouses) and level 3 functions (plant and warehouse operations management).

Profitability: Fully Automated Serialization vs. Manual Serialization
In his thesis on the subject, Efficiency Analysis of a Standard Track-and-Trace Software for the Pharmaceutical Industry, Zaim Imeraj performed a profitability analysis for serialization solutions. For that purpose, he compared fully automated serialization through the example of Werum’s PAS-X track-and-trace with a manually integrated serialization. In doing so, he identified all processes and calculated the related costs.
As an empirical survey, 11 packaging lines with an annual serialization of approximately 3,400 batches were used as a basis. Finally, Zaim evaluated the profitability of both solutions using methods of investment analysis.
Result: In the PAS-X scenario, the break-even point is reached at a very reasonable four months. In comparison with a manual serialization, a fully automated serialization will generate annually increasing savings amounting to millions. These savings result from shorter production times, compared to manual serialization, leading to increased profits.
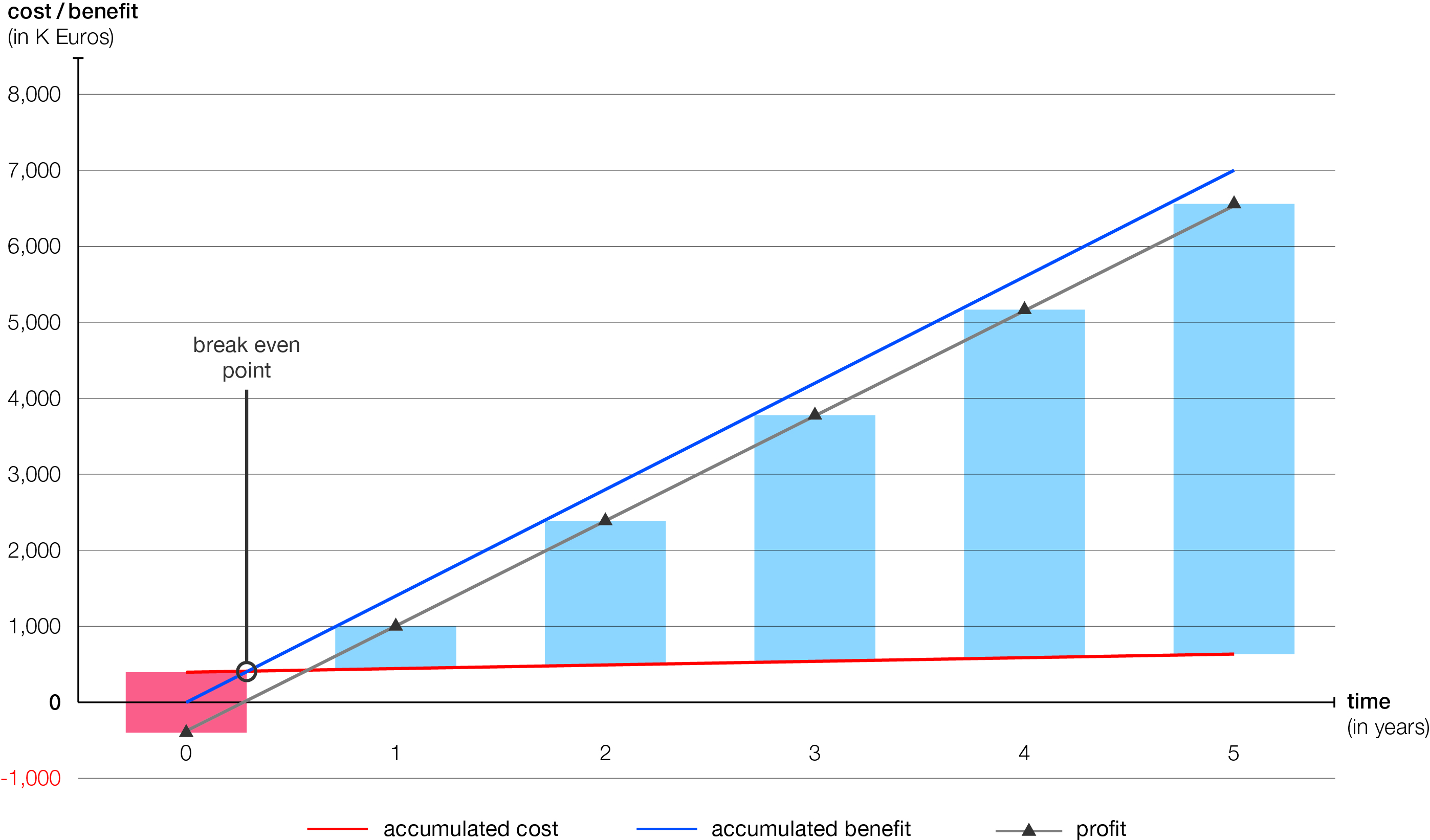
Fully automated serialization solutions turn out to be profitable after just a few months.
Summary
To fulfill the legal requirements for combating counterfeit medicines, pharmaceutical and biopharmaceutical companies must introduce track-and-trace solutions that provide all necessary features for the serialization and aggregation of medical products in packaging processes.
An efficient track-and-trace solution integrates the ERP system and the Central Repository at level 4 with the packaging equipment and line controllers on shop floor level 2. The serialization solution can be integrated into an MES to benefit from the advantages of comprehensive production control and documentation, including equipment management.
This creates a track-and-trace solution that allows for viewing and, if necessary, correcting serialization data prior to its transfer to the warehouse management system. Additionally, it enables managing serialization trees that branch out across the entire manufacturing area—such as repackaging and rework—and thus presents an alternative to level 4 systems.
This article can also be found in the September/October 2016 issue of Pharmaceutical Processing.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




