The Biden administration recently announced efforts to strengthen the pharmaceutical supply chain, mitigate shortages of essential medicines, and expand domestic manufacturing. The new efforts are part of an overall package of initiatives to boost U.S. supply chains that the White Houe announced on Nov. 27. Securing the country’s supply of medicines and lowering prices have…
U.S. government earmarks $1.6 billion to battle opioid crisis and overdose epidemic
The U.S. Department of Health and Human Services (HHS) has awarded more than $1.6 billion as a part of an initiative to battle the drug overdose epidemic. SAMHSA’s State Opioid Response (SOR), Tribal Opioid Response (TOR) grant programs and Health Resources and Services Administration (HRSA)’s rural communities opioid response programs will lead the new initiative.…
7 core strategies in Biden’s battle against COVID-19
Included in President Joe Biden’s sprawling strategy document for dealing with the COVID-19 pandemic are an array of plans intended to ramp up vaccination efforts. Also in the plan are proposals for accelerating the development of therapies such as antiviral compounds effective against SARS-CoV-2 and other coronaviruses with pandemic potential. The “full-scale, wartime” plans have considerable relevance…
U.S. government stockpile of COVID-19 vaccines already drained
Last week, President-elect Joe Biden vowed to release COVID-19 vaccine doses from a government stockpile. On Jan. 13, the U.S. Department of Health and Human Services (HHS) echoed that sentiment, stating it would “no longer stockpile millions of COVID-19 vaccine doses held to ensure Americans receive their second shot,” according to a brief notice. But federal…
BREAKING: Pfizer-BioNTech vials hold more COVID-19 vaccine doses than expected, according to FDA
Pharmacists have discovered that some vials containing COVID-19 vaccine from Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX) have up to 40% more doses than the five expected. Some pharmacists had thrown the leftover vaccine away out of fear of violating FDA policies, according to Politico. But FDA confirmed today that pharmacists can use the full amount of vaccine from the…
HHS chief assures smooth transition to Biden administration, COVID-19 vaccine availability
Health and Human Services Secretary Alex Azar stated on CNN that he has met with President-elect Joe Biden’s transition team. “I have already met with the Biden transition team. We want to make sure they get everything that they need,” Azar said in an interview with CNN’s Alisyn Camerota. Azar also noted that he would…
5 takeaways from the latest White House Coronavirus Task Force meeting
The White House Coronavirus Task Force promised a swift rollout of COVID-19 vaccines — but one of a number of takeaways from its first briefing since July. The briefing yesterday gave the task force a chance to once again urge Americans to heed COVID-19 guidance while also providing an update on vaccines and therapeutics. The…
What a Biden administration could mean for the pharma industry
While President Trump continues to refuse to concede the election, a growing number of business associations and politicians have acknowledged Biden as the president-elect. What a Biden presidency would mean for the pharmaceutical industry continues to be an open question as the Senate outcome remains undecided, but two broadly similar outcomes are likely. Ultimately, neither…
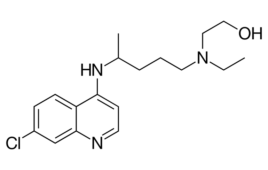
Study: Hydroxychloroquine not warranted for hospitalized COVID-19 patients
A National Institutes of Health clinical trial has concluded that hydroxychloroquine had no clinical benefit for hospitalized COVID-19 patients. The Journal of the American Medical Association published the results from the blinded, placebo-controlled, randomized trial. The clinical trial, known as the “ORCHID” study, treated 479 symptomatic COVID-19 patients with hydroxychloroquine in 34 hospitals in the…
HHS offering COVID-19 vaccine supply kits
The U.S. Department of Health and Human Services (HHS) recently announced it was gathering supply kits to facilitate a potential mass vaccine effort. FDA has yet to approve a vaccine for COVID-19, and at least five states are planning to require independent verification of vaccines after FDA approval. In the interim, HHS is partnering with…