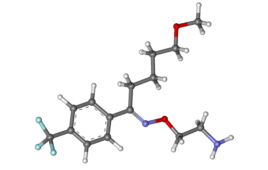
The infectious disease physician-scientist Dr. David R Boulware filed for the emergency use authorization (EUA) of fluvoxamine, an established selective serotonin reuptake inhibitor (SSRI) whose brand name is Luvox. The FDA has rejected the application, explaining the COVID-19 treatment benefit of the drug was “not persuasive” in the TOGETHER study that was the basis of the…