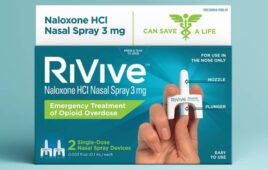
Harm Reduction Therapeutics announced that it received FDA approval for its over-the-counter RiVive naloxone nasal spray. Pittsburgh-based HRT designed the 3 mg naloxone HCl nasal spray for the emergency treatment of opioid overdose. Approval helps make free or low-cost OTC nasal spray widely available in the U.S. The FDA first approved Narcan 4 mg OTC…
5 core trends in drug recalls: An analysis of 2022 and Q1 2023
Drug recalls surged in 2022, with defective pharmaceutical units reaching a five-year high, according to the Sedgwick 2023 State of the Nation Recall Index. Also in 2022, sterility was the top cause of recalls for the first time in more than six years. January 2023 saw a significant increase in the number of drug recalls…
Vicks Nyquil FDA compliance issues explained
Procter & Gamble (NYSE:PG) recently received a warning letter from FDA for failing to meet the agency’s requirements in the drug listing. The warning related to Vicks Nyquil Severe Hot Remedy Cold and Flu Plus Congestion (National Drug Code (NDC): 58933-541), a powdered medicine intended to be dissolved in hot water before use. The FDA’s…
FDA releases guidance on pharmaceutical supply chain security
The FDA recently published guidance to help the pharma industry detect suspicious and illegitimate products within the pharmaceutical supply chain. These regulations, established under the Drug Supply Chain Security Act (DSCSA), concentrate on product tracing, verification, and identification for specific drug products distributed in the U.S. So far this year, the agency has released more than…
FDA ordered to ramp up unannounced foreign inspections
The omnibus spending bill recently signed by President Joe Biden directs the FDA to expand unannounced foreign facility inspections and evaluate them through a new pilot program. The legislation includes $10 million for the program and a mandate to increase unannounced surveillance inspections of foreign drug establishments, Regulatory Focus reports. The legislation also directs the…
FDA warns four companies over selling honey tainted with prescription drugs
FDA has sent warning letters to four companies for illegally marketing honey-based products with unlisted active drug ingredients found in the erectile dysfunction drugs Cialis (tadalafil) and Viagra (sildenafil). The agency said it confirmed the presence of contaminants in the products through laboratory testing. The agency sent the warning letters to the following companies: Thirstyrun…
First Zantac trial slated for February 2023
The California State Court Judge overseeing litigation related to Zantac (ranitidine) issued a pretrial order scheduling the first of four bellwether trials for Feb. 13, 2023, in Oakland. Honorable Judge Evelio M. Grillo for the California Superior Court of Alameda County will preside over the litigation. Before the cases head to trial, Judge Grillo will…
FDA prevented 317 drug shortages in 2021
FDA worked with pharma companies to avert a record number of drug shortages in 2021 based on a recent report to Congress that tracked data starting in 2012 when the FDA Safety and Innovation Act (FDASIA) was passed. In 2021, 115 manufacturers notified the FDA of 777 potential drug and biologic shortages. Ultimately, the agency worked to…
Young children in U.S. now eligible for COVID-19 vaccination
Some 18 months after adults became eligible for COVID-19 vaccines, children between 6 months and 5 years old can now be vaccinated in the U.S. FDA authorized the shots for young children on June 17, while CDC did so a day later. “The United States is now the first country in the world to offer safe…
Emergent hid quality problems from FDA, House report concludes
A new congressional report concluded that Emergent BioSolutions (NYSE:EBS) attempted to hide evidence related to quality problems before informing the FDA that 15 million COVID-19 vaccines were contaminated. Prepared for Rep. Carolyn Maloney (D-NY) and James E. Clyburn (D-SC), the report from the House and the Select Subcommittee on the Coronavirus Crisis scrutinizes the quality controls in…