FDA has authorized a second booster dose of COVID-19 vaccine for individuals who are immunocompromised or 50 and over. The expanded emergency use authorization applies to the vaccines from Pfizer (NYSE: Pfizer) and Moderna (Nasdaq: MRNA). Individuals interested in obtaining a second booster dose must wait at least four months after receiving the first booster dose.…
5 key steps for building a secure cold chain to maintain vaccine and therapeutic stability
COVID-19 vaccine development and rollout were crucial to gaining control of the pandemic. When the success of these efforts became dependent on the ability to maintain lower than typical temperatures during distribution, the importance of reliable and continuous cold chain solutions for mRNA-based formulations was thrust into the spotlight. Beyond pandemic mitigation measures and outside…
FDA advisory panel to convene to consider second booster dose
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) is scheduled to meet on April 6 to discuss the potential use of COVID-19 vaccine booster dose. Both Pfizer (NYSE:PFE) and Moderna (Nasdaq:MRNA) are seeking to amend the emergency use authorization of their respective vaccines to allow for an additional booster. Pfizer and its partner BioNTech SE (Nasdaq:BNTX)…
How to improve quality assurance in the pharmaceutical industry
The pharmaceutical industry is expected to grow at an annual rate of 5-8% for the next three years. In just the past five years, a range of new technologies has been implemented to improve healthcare delivery, analytics and drug production. However, with the lingering challenges of COVID-19, the need for creative solutions has become more…
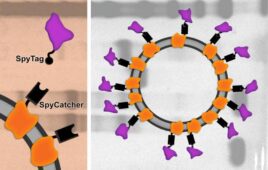
Engineered yeast could be key for more affordable COVID-19 vaccines
Researchers at MIT and Beth Israel Deaconess Medical Center are exploring a yeast-based alternative to RNA vaccines. A new paper highlights a vaccine, which comprises fragments of the SARS-CoV-2 spike protein arrayed on a virus-like particle, that reportedly elicited a strong immune response and protected animals against viral challenge, according to a post on MIT’s…
Countries in need will have access to Moderna COVID-19 vaccine tech
Moderna (Nasdaq:MRNA) announced four new initiatives within a global public health strategy for advancing mRNA vaccines. Cambridge, Massachusetts-based Moderna plans to expand its global public health portfolio, accelerate research in an effort to advance additional vaccines, expand its patent pledge to never enforce COVID-19 patents in the Gavi COVAX AMC for 92 low- and middle-income…
Johnson & Johnson inks agreement to have its COVID-19 vaccine manufactured in Africa
Johnson & Johnson (NYSE:JNJ) announced today that it completed an agreement for the manufacturing of its COVID-19 vaccine in Africa. The landmark agreement, made between Johnson & Johnson’s Janssen Pharmaceuticals and South Africa-based Aspen SA Operations, allows for the first COVID-19 vaccine to be manufactured and made available by an African company for people living…
WHO prequalifies aIL-6R therapy for patients with severe or critical COVID-19
Roche (SWX:ROG) has won World Health Organization (WHO) prequalification for Actemra/RoActemra. Actemra/RoActemra is an anti-interleukin-6 receptor (aIL-6R) receptor antagonist with tocilizumab as an active substance. The prequalification will assist in low- and middle-income countries in procuring the therapy for patients on systemic corticosteroids who require supplemental oxygen or mechanical ventilation. Actemra/RoActemra is the twelfth medicine…
Addressing vulnerabilities in the medical product supply chain
While the medical product supply chain has always been subject to vulnerabilities, those weaknesses were laid bare early in the COVID-19 pandemic. Drug and medical device supply chains have historically been susceptible to factors such as a lack of supplier redundancy, components, active pharmaceutical ingredient (API) or finished product manufacturers. But pandemic-driven sourcing and workplace…
FDA fully approves Moderna’s COVID-19 vaccine
Moderna (NSDQ:MRNA) announced today that it received full FDA approval of the biologics license application for its COVID-19 vaccine. FDA’s approval for the company’s Spikevax mRNA COVID-19 vaccine covers the prevention of the virus in individuals aged 18 years or older. Moderna submitted for full FDA approval back in June 2021 and becomes the second…