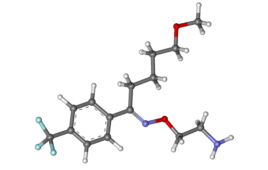
Pfizer (NYSE: PFE) announced today that it will invest $120 million into its Kalamazoo, Michigan facility to support U.S.-based production of its COVID-19 antiviral Paxlovid (nirmatrelvir/ritonavir). The investment is expected to create more than 250 additional, high-skilled jobs at the Kalamazoo site. The money will expand the production of active pharmaceutical ingredient (API) and registered…
FDA denies EUA for COVID-19 indication of SSRI fluvoxamine
The infectious disease physician-scientist Dr. David R Boulware filed for the emergency use authorization (EUA) of fluvoxamine, an established selective serotonin reuptake inhibitor (SSRI) whose brand name is Luvox. The FDA has rejected the application, explaining the COVID-19 treatment benefit of the drug was “not persuasive” in the TOGETHER study that was the basis of the…
Pfizer ticks up on Street-beating Q1, lessens full-year guidance
Pfizer (NYSE:PFE) shares rose today on first-quarter financial results that came in ahead of the consensus forecast. The New York-based pharmaceutical company posted profits of $7.9 billion, or $1.37 per share, on sales of $25.7 billion for the three months ended April 3, 2022, for a 61.2% bottom-line gain on sales growth of 76.8%. Adjusted…
Johnson & Johnson rises on mixed bag Q1, increased 2022 guidance
Johnson & Johnson (NYSE:JNJ) shares ticked up today on first-quarter results that were mixed compared to the consensus forecast. The New Brunswick, New Jersey-based company posted profits of $5.1 billion, or $1.93 per share, on sales of $23.4 billion in the first quarter for a 16.9% bottom-line slide on sales growth of nearly 5%. Get the…
NIH starts evaluating second COVID-19 booster shots in adults
The NIH recently announced that it has started enrolling adults for a Phase 2 clinical trial evaluating various additional COVID-19 booster shots. NIH’s National Institute of Allergy and Infectious Diseases (NIAID) is sponsoring the COVID-19 Variant Immunologic Landscape (COVAIL) trial. “We are looking beyond the Omicron variant to determine the best strategy to protect against…
Engineered yeast could be key for more affordable COVID-19 vaccines
Researchers at MIT and Beth Israel Deaconess Medical Center are exploring a yeast-based alternative to RNA vaccines. A new paper highlights a vaccine, which comprises fragments of the SARS-CoV-2 spike protein arrayed on a virus-like particle, that reportedly elicited a strong immune response and protected animals against viral challenge, according to a post on MIT’s…
Countries in need will have access to Moderna COVID-19 vaccine tech
Moderna (Nasdaq:MRNA) announced four new initiatives within a global public health strategy for advancing mRNA vaccines. Cambridge, Massachusetts-based Moderna plans to expand its global public health portfolio, accelerate research in an effort to advance additional vaccines, expand its patent pledge to never enforce COVID-19 patents in the Gavi COVAX AMC for 92 low- and middle-income…
Johnson & Johnson inks agreement to have its COVID-19 vaccine manufactured in Africa
Johnson & Johnson (NYSE:JNJ) announced today that it completed an agreement for the manufacturing of its COVID-19 vaccine in Africa. The landmark agreement, made between Johnson & Johnson’s Janssen Pharmaceuticals and South Africa-based Aspen SA Operations, allows for the first COVID-19 vaccine to be manufactured and made available by an African company for people living…
FDA fully approves Moderna’s COVID-19 vaccine
Moderna (NSDQ:MRNA) announced today that it received full FDA approval of the biologics license application for its COVID-19 vaccine. FDA’s approval for the company’s Spikevax mRNA COVID-19 vaccine covers the prevention of the virus in individuals aged 18 years or older. Moderna submitted for full FDA approval back in June 2021 and becomes the second…
Generic manufacturers to make cheaper version of Merck’s COVID-19 pill for developing countries
The Medicines Patent Pool (MPP) announced today that it signed agreements with 27 manufacturers to produce Merck’s COVID-19 pill. MPP’s agreements with the generic manufacturing companies cover the manufacturing of the oral COVID-19 antiviral medication molnupiravir, with supply set for 105 low- and middle-income countries (LMICs). The agreements come as a result of the voluntary…