Gene and viral-vector-modified cell therapies are areas of development generating tremendous excitement in the pharmaceutical industry today. The first US FDA approvals were granted in 2017 to the chimeric antigen receptor (CAR) T-cell immunotherapies Kymriah (tisagenlecleucel) from Novartis and Yescarta (axicabtagene ciloleucel) from Kite Pharma (a Gilead Company) and the adeno-associated viral vector-based gene therapy Luxturna (voretigene neparvovec-rzyl) from Spark Therapeutics. Hundreds of additional candidates are in early to late-stage clinical trials and show great promise to positively impact the lives of millions of patients.
Widespread commercialization of gene and modified cell therapies will be challenging; however, much remains to be established with respect to practical and scalable manufacturing platforms. To produce viral vectors, the appropriate cells must first be grown and then transfected, typically using a plasmid formulation. The viral vector is then harvested from the cells and formulated for use, either directly as a gene therapy or for the medication of patient cells, such as in CAR-T cell therapies.
While monoclonal antibodies and other conventional protein-based biologics are typically manufactured using suspension cell culture processes, some of the viral vectors required for the production of gene and modified cell therapies are produced via adherent cell culture. Manufacture of adenoviral vectors, which are one of the common virus types used to deliver therapeutic genes, requires the conditions provided by adherent cell culture. In adherent cell culture, the cells are supported on a matrix of some type, with the two most common options being microcarriers and multi-tray static plasticware.
Microcarriers made of materials such as glass, plastics (polystyrene, acrylamide) or natural polymers (collagen, alginate) have properties that promote cell growth. To maintain a homogeneous environment for the adhered cells, the microcarriers are suspended and agitated in the bioreactor. For the shear-sensitive cells required for viral vector production, however, this agitation can be damaging. In addition, because the hydrodynamic conditions within the bioreactor change with process volume, scale up of microcarriers processes can be quite challenging. Achieving high cell densities is often challenging, so processes that can be successfully scaled up generally require large quantities of expensive transfection agent.
Given these issues with microcarriers, most adherent cell culture processes for adenoviral vector production involve the use of static plasticware, which can include flasks, roller bottle, cubes or multilayer cell factories. Two issues with these systems are a lack of precise control over environmental conditions, including pH, dissolved oxygen (DO), and media composition and the limited ability to achieve manual intervention during the process.
Perhaps the greatest challenge, however, is the inability to scale up plasticware—the only option is to scale out by performing multiple processes in parallel.
Static plasticware has been perfectly adequate for discovery and development efforts to date, and even for the production of early clinical trial materials. It is not, however, practical for achieving cost-effective, timely production of the large quantities of viral vectors that will be needed as many gene and modified cell therapy candidates move to the commercialization stage.

Figure 1: Scale-X carbo bioreactor system. Image: Univercells
A Scalable Fixed-Bed Reactor Alternative
Univercells developed the scale-X bioreactor portfolio of single-used fixed-bed bioreactor technologies (see Figure 1) for intensified cell culture and viral production. The portfolio is automated, scalable from lab to commercial production, and cost-effective. The portfolio includes the hydro system (2.4 m2), carbo (10 to 30 m2), nitro (200 to 600 m2) and oxo (>2000 m2) to support process development, pilot scale, medium-to-large-scale industrial production (typically for vaccines), and larger scale industrial production of gene and modified cell therapies, respectively.
Within the fixed bed, a tightly packed support matrix consisting of spiral-wound, nonwoven polyethylene terephthalate (PET) fabric layers allows for high cell densities in a small-footprint bioreactor. The fixed bed is combined with a magnetic centrifugal impeller inside the bioreactor. This impeller provides good mixing to ensure even availability of nutrients throughout the fixed bed and aeration via the creation of a “falling film” in the vessel headspace to increase the surface area available for gas exchange.
This design results in conditions similar to those observed in static plasticware, with gentle circulation of the media across and through the cells. The important difference is the homogeneity of the environment in both the vertical and horizontal directions. Scale up is achieved by increasing the height and diameter of the fixed bed in parallel so that the linear velocity of liquid media travelling through the fixed bed remains constant across scales and, as a result, the cells experience similar low-shear conditions.
The inclusion of automated pH, DO and temperature control capabilities enable the development of robust, high-yielding processes. Automated inoculum addition, manual media and cell sampling capabilities, and pumps that allow further media addition through a recirculation loop round out the design features of the bioreactor.
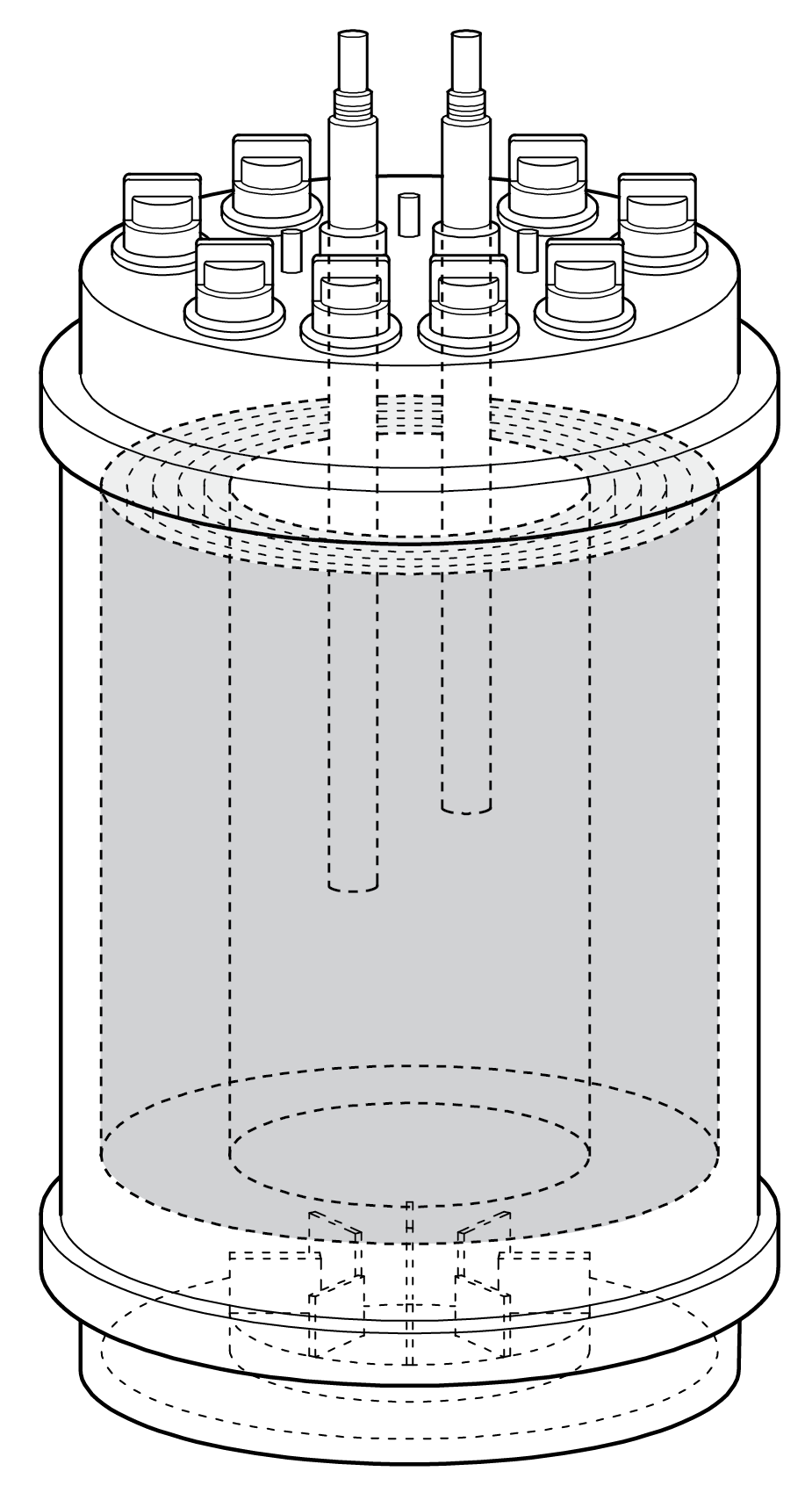
Figure 2: The bioreactor features a structured design. Image: Univercells
Process Transfer
The scale-X fixed-bed bioreactor design is built for successful process transfer from established plasticware-based viral vector production processes involving adherent cell culture. To demonstrate this feature, the performance of processes conducted in the scale-X hydro bioreactor system and a standard plastic flatware cell culture flask were directly compared using the same inoculation density and proportional volume of culture medium for each.
For this study, HEK293 cells from a cryopreserved cell bank (Momotaro-Gene) were used. The inoculum was generated in a cell culture flask under standard conditions. The processes were monitored for cell density and virus titer by recording visual counts using an optical microscope and glucose and lactose metabolite concentrations using an off-line metabolite analyzer.
Three different processes were run in scale-X hydro bioreactors. For all of these processes, media was supplied via an external media source connected during cell expansion and recirculated through the bioreactor shortly after inoculation (4.2 L). In bioreactor #1, the media was replaced at day 3 to allow further cell growth, but the cells were not infected. In bioreactors #2 and #3, cell expansion was performed in batch mode for 2 and 3 days, respectively, after which time the cells were infected. The five steps that take place during cell culture in the scale-X hydro bioreactor (bioreactor preparation, inoculation and cell attachment, cell expansion, viral infection, harvest) can be seen in Figure 2.
The results for cell growth are presented in Figure 3. It can be clearly seen that in bioreactor #1, the cell density was higher than that observed in the static plasticware at days 3 (2.1 × 105 vs. 1.3 × 105 cells cm–2) and 6 (6.5 × 105 vs. 3.2 × 105 cells cm–2) under the same conditions, which included the same ratio of medium exchange. Cell growth in the fixed-bed bioreactors #2 and #3 was also higher.
In bioreactor #1, the external media circulation loop was replaced with fresh medium, thus allowing further cell growth until day 6. In bioreactors #2 and #3, the processes were run in batch mode, with infection taking place on day 2 and 3, respectively. Note that the cell density post-infection is not shown, and the control cell density for bioreactor #2 was not available.
Infection was performed on days 2 and 3 (bioreactors #2 and #3, respectively) at a target cell density. In all cases, the infected cells were found to be lysed 3 days post-infection. Although the cells naturally lyzed, product recovery was enhanced using a detergent treatment. Despite the lack of any process development work, the viral titer in the fixed-bed bioreactor was only approximately 1 log IRU less than that in the control plastic flatware.
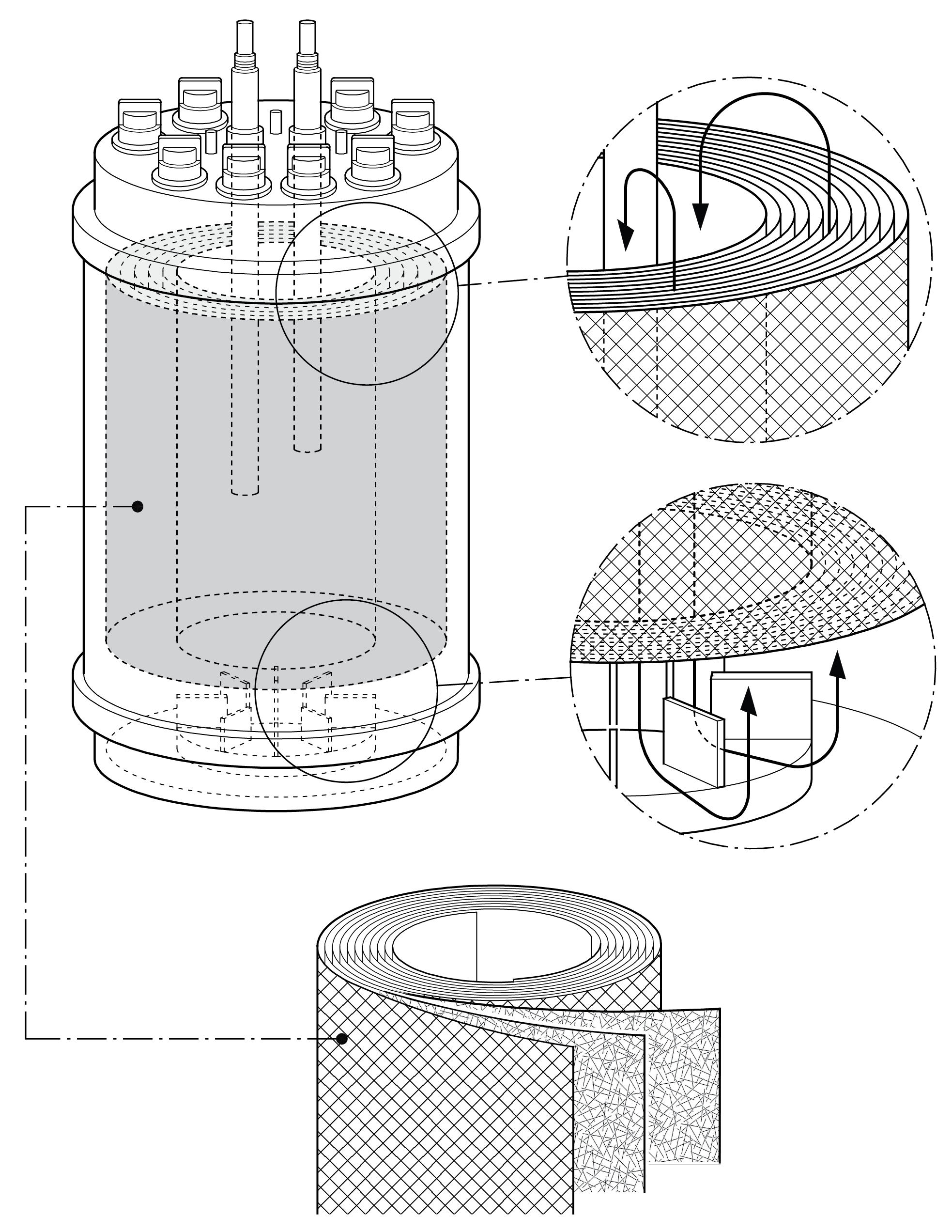
Figure 3: The fixed-bed ensures a homogeneous media flow. Image: Univercells
Future Work
The results of this comparison study clearly demonstrate the ease with which an HEK293 process producing an adenovirus for gene therapy can be transferred from static plasticware to the new scale-X hydro bioreactor. High cell densities and good viral yields were achieved within a small bioreactor volume with no process optimization. The new fixed-bed bioreactor has therefore been shown to have the capability for high levels of production in a much-reduced footprint, with the added benefit of increased automation. The higher cell densities in the fixed-bed bioreactor are likely due to the ability to achieve a stable growth environment due to the enhanced control of pH, DO and other process conditions.
The next steps will include determination of the critical process parameters (CPPs) for optimization of the culture conditions in the fixed-bed bioreactor. This type of process optimization is not possible in staticware processes due to the lack of control over pH, DO and other factors afforded by such equipment. Further studies will also be performed to demonstrate the scalability of the process across the full portfolio of scale-X bioreactors.
Alex Chatel is product manager at Univercells. In his early career, Alex spent time working and teaching at the University College of London (UCL), and was a research engineer at GlaxoSmithKline. His educational background includes a Doctorate of Engineering from UCL, as well as degrees in biochemical engineering and business management.




