Exclusive Q&A with GlobalVision on print inspection best practices in the pharmaceutical industry.
Operations in the pharmaceutical industry are complex—and rightly so. Pharmaceutical packaging is no exception. Vast amounts of information are required to be present on any given pharmaceutical product. But with so much information squeezed into that tiny space, there is always the risk that the packaging could potentially harbor a number of errors that can render the product defective and mandate its removal from the market.
Mike Malz, Marketing & Creative Director of GlobalVision, participated in an exclusive Q&A with Pharmaceutical Processing on best practices for print inspection in the pharmaceutical industry. His edited responses are below.
To start, what are the various elements that must be displayed on a pharmaceutical package (cartons, labels, and package inserts)?
Malz:
The most important element to display on pharmaceutical packaging is the FDA-approved copy which includes legal notices, directions for use, and disclaimers. The FDA approves the copy as part of the regulatory process and must appear exactly as is on the final package—the slightest difference can mean a recall from the market.
Graphics can be included, particularly in the case of branded products vs. generic alternatives. It’s also often used in instructions for use to show the user how to use the product, particularly for injectables and medical devices.
Barcodes are another important element in packaging. Pharmaceutical drugs are expensive products and (consequently) prone to counterfeiting. To ensure patient safety, barcodes are used to encode unique serial numbers and lot numbers to ensure drugs are tracked and traced to guarantee they are the original product. The pharmaceutical barcode is the primary barcode and serves as the key identifier of the drug. This is followed by the UPC code which is the key identifier for point-of-sale. Other barcodes may also be present for print production, tracking, and ID. We rely heavily on barcodes in today’s marketplace, and the use of multiple barcodes on a pharmaceutical package makes for an element that proves most complex, and crucial to manage.
Finally, Braille is a key component of pharmaceutical packaging in European markets, where it is mandatory to include the product names for the blind with the EU Directive 2001/83/EC.

What errors most commonly occur in pharmaceutical print inspections (specifically with regards to text verification and quality assurance)?
Malz:
The packaging element that is most prone to errors is copy. It is the most important information from a regulatory perspective and requires the greatest amount of time and detail when performing quality assurance inspections.
Surprisingly, the most common error that surfaces in copy is spelling. The chemical and technical terms in the industry can reach upwards of 35-40 characters long. Often, graphic designers who typeset the copy into artwork for print do not run spellcheck because it is not their responsibility or because the tools are not made available. Verifying the spelling of Ibuprofen, Ventolin, Polysporin, Nyquil, or Methylprednisolone can prove to be a taxing daily challenge. Not only must the technical terms be perfect, but so does the numerical information. A mistake of “Take every 2-4 hours” vs. “Take every 24 hours” is an easy difference to miss when verifying text, but as you can imagine it’s an error that cannot happen.
“The packaging element that is most prone to errors is copy. It is the most important information from a regulatory perspective and requires the greatest amount of time and detail when performing quality assurance inspections.”
Errors also occur when proofreading English copy. It’s easy if English is your first language, but it becomes challenging when looking at multi-lingual packaging with languages you can’t read. And regulations today call for more languages to be included in print (not less). For Canada, this includes French, whereas in the U.S., this can include Spanish. But in Europe with different countries and languages spoken, it’s common to have three languages or more in a patient information leaflet.
These elements reveal a strong theme across common errors during print inspections—lack of readability. People performing inspections rarely understand what they’re inspecting because they don’t read the language. This theme extends to Braille, too, where quality assurance personnel usually cannot read it. It only takes one dot to change a product name or dosage; therefore, the level of detail required in verification is extraordinary.
“These elements reveal a strong theme across common errors during print inspections—lack of readability.”
Lack of readability is even more present in text elements independent of language. The symbols most commonly used in pharmaceutical packaging such as mL, mg, Epsilon, MU, superscripts, and subscripts are also common errors that occur. With just one extra key press, you can change mg to mcg which alters the dosage of the medication. The differences of symbolism are too easy to mix up.
This goes for barcodes, too. You need to be able to first tell during inspections if the correct barcode was placed on the product and then if it’s of sufficient quality based on ISO standards so it can be scanned.
What are some of the common causes for print inspection errors and how can they be avoided?
Malz:
The best way to stop spelling mistakes is not obvious. You need to get someone to own the problem. It’s usually hard to find an owner for this task, especially pre-press or graphics. Using automation tools for spellcheck can help to perform an automated proofread of the document so that potential errors can be presented to the quality assurance department.
Copy errors can be avoided by proofreading, either manually or using automated pre-press tools, and comparing copy text from the customer’s copy vs. the artwork file to ensure proper typesetting.
Barcode errors can be avoided using barcode scanners and verifiers to check quality and barcode data against the job data. Barcode checks can be performed using offline verifiers, inline verifiers, or even in pre-press directly on a PDF or AI file.
With Braille, having standards for each Braille language, having the quality assurance personnel check each dot pattern, or using pre-press verification tools for Braille can help ensure that it matches the job information from the order. Measurement tools such as a caliper or mechanical scanner can be used to check that the height requirements for European Braille are met.
Also, just like having 200 or 300 percent proofreading where you have multiple people proofreading the same documents to check all types of errors, it’s important to implement different types of inspection throughout the packaging artwork and printing process. Having a variety of methods in your process can increase your chances of catching errors. Implement checks at various stages of the artwork process—including text typesetting, customer proofing, pre-press, step and repeat artwork creation, plate file creation—all the way to press setup with offline print inspection, and production inspection with inline print inspection. The more checkpoints you have, the less likely an error will get by. Furthermore, the cost of correcting these errors is much lower if they are found at the beginning of the process.
Of course, adding more checkpoints requires more man hours as well, so it’s best to use technology such as pre-press content verification tools and inspection solutions to assist operators and reduce manual time spent on these efforts. Newer technologies, including automated workflows for quality assurance, promise to automate routine quality checks in the background to save additional time and improve accuracy.

What are some offline print inspection best practices?
Malz:
- Have a check in place to make sure you have the right PDF file and that it’s the latest version.
- Verify the approved PDF vs. the printer’s proof before the approval is given to the printer to begin production.
- Printers should print by the sheet or web and not one pack at a time.
- Make sure you inspect the approved PDF file vs. one sheet so that all print positions get checked.
- Do this check at least once at the beginning and at the end of the print run.
- Look for software solutions for print inspection. Companies need to be aware that the printing process is not perfect. Every print job needs to be inspected even if the last one had no issues.
What are the best practices for inline print inspection systems?
Malz:
Make sure the approved customer PDF file is introduced into the inline inspection system. Otherwise, you could be printing the wrong product and delaying delivery of your product to market.
The data from an inline print inspection system is overwhelming. Communicate to your printer where your critical areas are on your package. If you can let go of some areas for inspection or at least reduce them, you will be putting more horsepower in critical areas such as ingredient lists.
What inspections should a company perform prior to an error occurring? After an error occurs?
Malz:
Different checks can be applied in various stages in the process before printing to avoid errors being introduced along a step in your process. Identifying errors early minimizes the risk in terms of cost and time, avoiding reprints, claims, and delays to a customer.
In pre-press, content verification allows you to check text copy from the copy document vs. the typeset artwork. Graphics, colors, and images from the pre-press files can be compared to the customer approved PDF. Braille and barcodes can be verified on the PDF.
Imposition files and plate files, such as 1-bit TIFF and Esko LEN files, can be inspected to ensure the correct products are placed from the initial artwork files.
Using offline print inspection technologies ensures correct press setup before doing inline inspection during the production run.
If an error is detected, using inspection software and technology can help determine the number of affected batches in the job to see if some of the costs can be recouped.

How can a company optimize their print inspection processes in order to better identify any errors that occur?
Malz:
Every printed package is made up of what can seem like an endless number of elements, including copy, barcodes, logos, colors, dieline, graphics, symbols, nutritional facts, directions, legends, and more. So, to optimize the inspection process, it’s often best for the inspection system to automatically locate and divide the inspection tasks by these specific pack elements. Focusing inspections on one element at a time vs. everything at once often gives greater chances of discovering errors. For example: locate all of the barcodes automatically, then decode and grade each one, then append the results to the report.
Should a product be rejected, what are the next best practice steps that should occur?
Malz:
The best way to mitigate this, especially if printing for pharmaceuticals, is to have a CAPA (corrective and preventative action) process in place. CAPA systems are used routinely in quality management in pharmaceuticals and as a best practice should be extended to pharmaceutical printing—where a root cause analysis should be performed if an error does go by.
“Focusing inspections on one element at a time vs. everything at once often gives greater chances of discovering errors.”
The root cause analysis is a basis for a corrective action to avoid the same issue happening again. The “5 Why” method is common to try to find the root cause and implement the necessary corrective actions by asking the question “why?” five times for a problem that occurred.
Here’s an example:
A printing mistake went by. The copy was incorrect, and the customer rejected the order.
Why?
The hyphen was missing on the final print in the directions and this was not detected.
Why?
QA missed it during proofreading.
Why?
Joe was on vacation, and Chris was the one who was doing the proofreading but was not familiar with what to check.
Why?
The process is manual and not documented concerning what items and types of differences to look for.
Why?
The operators are so busy with the number of jobs to proofread that there wasn’t enough time allocated to spend documenting this.
The end result should lead to a corrective action which in this case would be to document standards for proofreading, or maybe alternatives such as an electronic system, so that the method is standardized on the way to look for errors.
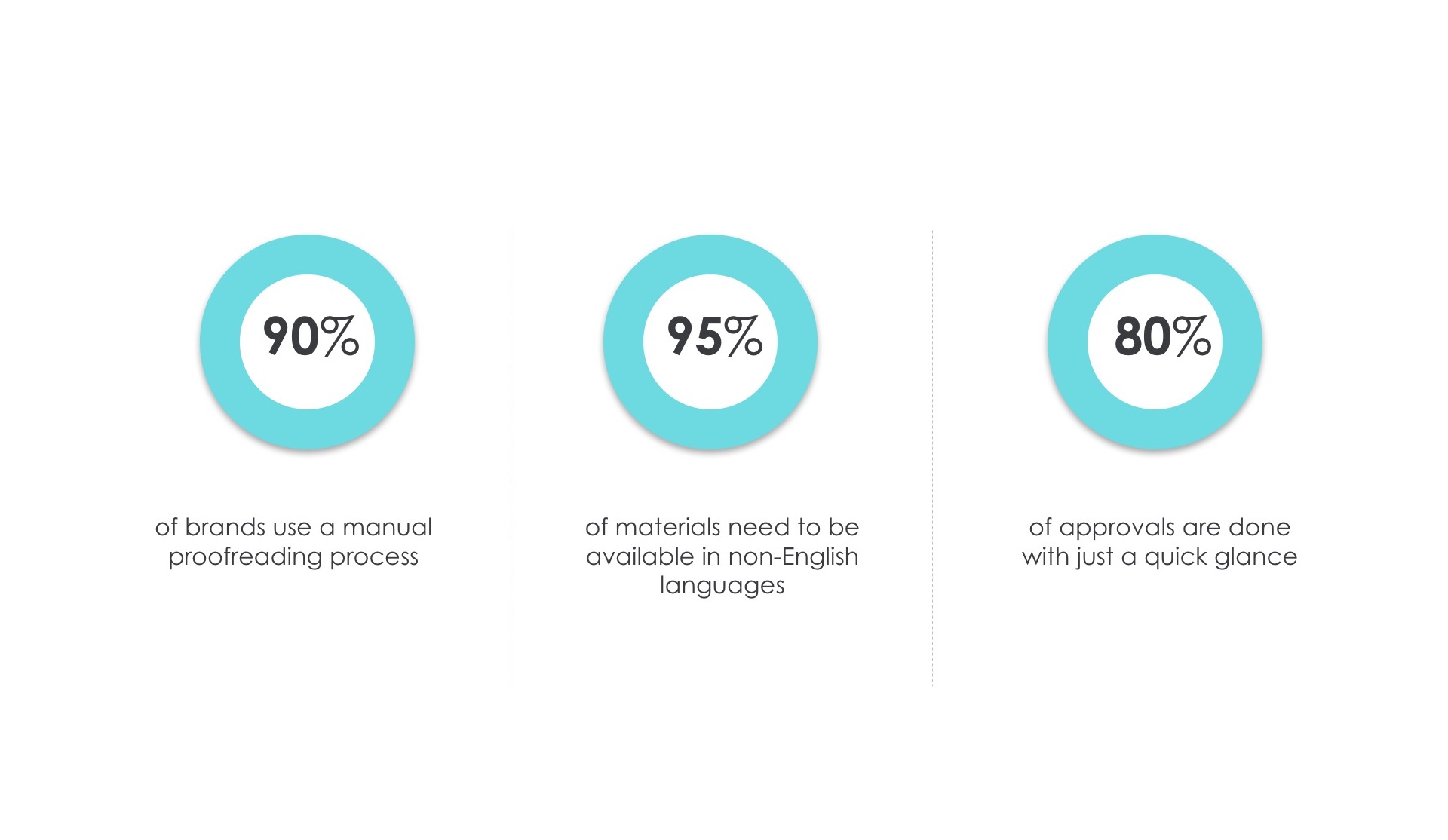
How much does color impact a print inspection?
Malz:
Each industry has different requirements and defines pack quality differently. For a cosmetic pack or beverage, color is paramount. For pharmaceutical, copy is critical.
Therefore, color is not impacted for most pharmaceutical packaging but consumer packaging is critical.
Another issue is background color and contrast. For pharmaceutical packaging and readability, colors in the background are often used minimally to make sure that text copy is easy to read, whereas in consumer industries colored background are acceptable and used to catch the attention of the consumer.
Are there certain pharmaceutical packaging materials that you have found don’t work well with certain products?
Malz:
Holographic print does not work well. Shiny materials such as foil, silver, or gold materials, recycled paper stock, and very thin paper (which can cause ink to bleed through to the other side) complicate the print inspection process. However, there are technologies and methods to deal with these with modern print inspection technology. It’s often a case of adapting print inspection methods to work with packaging materials as new ones are created and the industry changes.
How do you foresee the DSCSA (Drug Supply Chain Security Act) affecting print inspection in the future as the regulations are implemented across the industry?
Malz:
DSCSA mandates unique serial numbers encoded in barcodes for product traceability for patient safety. The main effect on print inspection is the focus on barcodes—particularly inline as the code are automatically generated and are unique so they must be checked dynamically.
Post-print, being able to verify barcodes automatically vs. databases is a validation step that requires print inspection software to connect to databases, and potentially, the cloud.
What are some of the print inspection trends you’re seeing? Are there new technologies that are becoming prominent across the industry?
Malz:
Emerging print inspection trends include remote setup monitoring and configuration reducing pressmen involvement. Inspection areas and objects are automatically selected, and inspection sensitivities and parameters are preset according to customer requirements before press production commences.
“Each industry has different requirements and defines pack quality differently. . . . For pharmaceutical, copy is critical.”
The customer is moving into the quality control process via web-based collaboration. The brand company can log in to the print process and approve, reject, and support his printer in real-time as the job is in production.
Other trends in the industry include full automation of routine quality checks. Being able to automate checks of pre-press files, step and repeats, barcodes, and spelling is key to helping companies deal with the pressure of more orders and jobs without sacrificing quality. This is a win-win especially for pharmaceutical printers, where quality is key in the selection of suppliers.
Connectivity is rising as well. If you look at a printer, you’ll have an MIS like CERM, ERP software, a customer portal with WebCenter, design tools like Adobe Illustrator, pre-press inspection software with GlobalVision, inline inspection with AVT, and pre-press workflow automation with Esko Automation Engine. The ability to connect these systems together in a workflow provides more time savings, as there is less time spent transferring files between different systems.
Imagine a scenario where the customer can upload a job in the portal, transfer it to the MIS, have the graphics artist create the file, check the content automatically, get the required changes direct in Illustrator, then send it off to production with the settings for the inspection system pre-defined in Illustrator. All of these systems are increasingly becoming connected, integrated, and automated. Collaboration between systems is creating a master process and is where technology for printing is headed—and it is already possible to start implementing to those interested in getting ahead of the curve.
This Q&A also appeared in the March 21 edition of the 2017 INTERPHEX Show Daily.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




