In 2016, pharmaceutical recalls went up in Q2—though, it was down compared to historical trends in 2004, according to Stericycle ExpertSOLUTIONS’ quarterly Recall Index.
“The Recall Index is a quarterly analysis of the ‘what’ and ‘why’ behind recall activity in the U.S.,” said Michael Good, Vice President, Stericycle ExpertSOLUTIONS. Focusing on five industries—pharmaceuticals, automotive, consumer products, medical devices, and food and beverage—the Recall Index compiles data from all of the major U.S. government agencies that order product recalls.
Revealing recall trends across numerous verticals and sectors, the Recall Index also found that 66 percent of recalled pharmaceutical units in Q2 were Class II and the average event size was approximately 110,000 units. Compared to 2014—where the average number of units per recall numbered just shy of 350,000—in Q1 and Q2 of 2016, the number of units per recall was only 82,549.
Good participated in a Q&A with Pharmaceutical Processing to discuss the findings of the Recall Index as well as trends in pharmaceutical recalls. His edited responses are below.
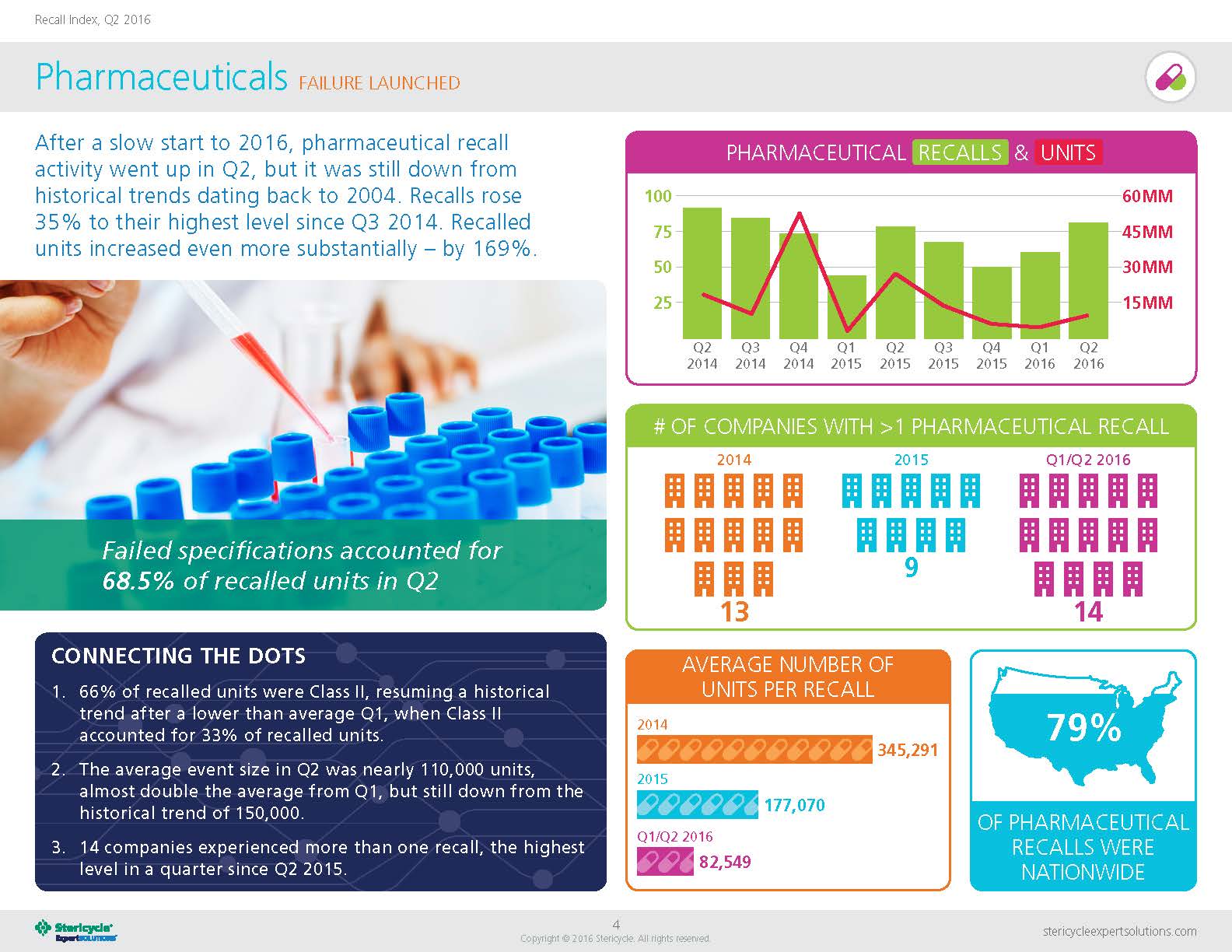
Photo courtesy of Stericycle ExpertSOLUTIONS; page four of its quarterly Recall Index
Could you give us a quick background on the Stericycle Recall Index?
Good: We launched the Recall Index in 2012 to provide industry and consumers with a broader view of recall activity and its causes. It also points out broader recall trends within each industry. In our Q2 2016 Recall Index, for example, we reported a steep increase in food recalls. The spike was partially due to better contamination detection methods, rather than a growing safety issue.
What time of the year do most pharmaceutical recalls take place, and why do you think that is?
Good: There isn’t really a time of year when pharmaceutical recalls—or any other recalls, for that matter—are more common. We find that pharmaceutical recalls tend to fluctuate from quarter to quarter; and while we know the causes of recalls, there isn’t always a clear reason for those peaks and valleys within the industry has a whole.
How do pharmaceutical recalls in 2016 compare to previous years? What are some of the biggest trends?
Good: There was an interesting anomaly in Q1 2016 numbers when Class III recalled units spiked above Class II. Class II went back into the top spot in Q2, but it was still curious because Class II had so consistently been the most heavily recalled category of pharmaceuticals.
Average recall sizes through the first two quarters of 2016 are significantly lower than the historical average of 150,000 units. That compares to 55,000 in Q1 and 110,000 in Q2. If that holds up through 2016, it could signal an emerging trend. Also, while Q2 saw an uptick in the number of pharma companies being hit with more than one recall per quarter, when combined with Q1 the overall trend in the first half of 2016, it is lower than the historical average.
What are the most common factors contributing to a pharmaceutical recall?
Good: Failed specifications were the top cause in Q2, accounting for 68.5 percent of recalled units. In Q1, mislabeling made up the majority of recalled units at 51 percent. This shift demonstrates that, just as the number and size of recalls vary widely from quarter to quarter, so do the most common causes.
In the Recall Index, there were statistics citing the number of companies that had more than one pharmaceutical recall. What were some of your key findings there, and how can companies work to have one (or zero) pharmaceutical recalls?
Good: Fourteen pharmaceutical companies experienced more than one recall in Q2—the highest number since Q2 2015. This is especially interesting given that mergers and acquisitions have resulted in fewer companies operating in the industry. Implementing additional quality control measures can certainly help reduce this number, but the reality is that even the most diligent companies will experience recalls. That’s why preparedness and continual improvement are so important.
What were some of the typical pharmaceutical products that often undergo a recall?
Good: The types of pharmaceutical products that undergo a recall are as varied as the available products on the market. They include over-the-counter, prescription, and compounded drugs ranging from tablets and capsules, injectable drugs, liquids, lotions and creams, and many more.
Financially, how much do pharmaceutical recalls set back any given company?
Good: Every recall is different, so the financial impact varies greatly. It’s based on a number of factors, including the number of units affected and the global reach of the recall. There are also indirect costs that need to be factored in. For example, if pharmaceutical companies don’t work with partners to execute the recall, their internal staff must step in at the expense of their usual duties.
What are some of the initial steps a pharmaceutical company can/should take once a product is recalled?
Good: If you don’t plan long before a recall hits then you’re already behind the eight ball when it does. Preparation is most of the game. Consult with a recall expert to draw up a management plan. Have a recall team in place to execute the plan. Train them and everyone else who’s going to be involved to make sure they understand their roles and can perform them. Run a mock recall to see what you do and don’t do well. Fix what you are doing wrong or not doing at all.
With your recall plan in place and your team ready to swing into action, you’re going to know the initial steps of the actual recall cold: identifying the affected products and where they were sold; notifying customers; and organizing product collection. Recalls don’t have to be scary, but without planning and careful preparation they can be. That’s because you’re going straight into unknown territory and there usually isn’t time to learn on the fly.
In your findings, were any specific types of pharmaceutical company frequently undergoing a recall of their product? If so/if not, explain.
Good: In every quarter we’ve analyzed there have been recalls from both major pharmaceutical companies that produce wide varieties of prescription and over-the-counter drugs and smaller compounding pharmacies that tailor drugs to individual patients. Recalls happen to all types of companies, which is why a robust recall plan is so crucial.
How do pharmaceutical recalls compare to recalls in other industries?
Good: Pharmaceutical recalls differ from many other recalls in that they may be at the hospital, retail, or consumer level, depending on the type of drug involved. These recalls are managed differently. While a hospital should have a procedure in place for locating affected product, patients and consumers do not, so these recalls are often handled similarly to a consumer product recall.
What is your advice for recall preparedness (should a pharmaceutical recall happen—how can companies best prepare beforehand)?
Good: Practice makes perfect in just about everything, and that is especially true for recalls. So pharma companies should definitely orchestrate a mock recall. An actual recall is a really poor time to figure out what everyone should be doing and how. When you do a mock recall, you’re not facing the unknown when your customers’ health and your reputation are on the line. And if that isn’t enough to convince you, most insurance carriers will give you a discount or rebate for conducting a mock recall, so it makes even more business sense.
How do you foresee different regulations, such as the DSCSA, impacting pharmaceutical recalls?
Good: Long term, DSCSA regulations will likely make recalls more precise and less costly. Getting to that point won’t be a lot of fun for the pharma companies, though, because the capital improvements they have to make to comply with the new regulations aren’t cheap. Implementing serialization on all of their production lines, for example, requires specific engineering experience that most pharma companies and contract manufacturing and packaging organizations (CMOs/CPOs) just don’t keep in house. Once they get through that pain, though, reaching out to those customers responsible for retrieving the product will be faster, more precise, and cost effective.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




