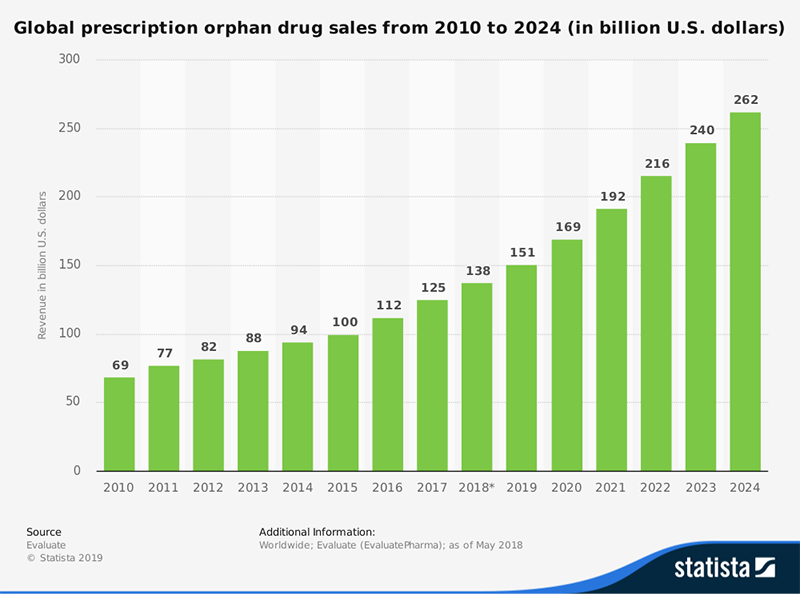
Globally, $125 billion worth of orphan drugs targeting rare diseases sold in 2017. By 2024, that number is projected to reach $262 billion, growing at a CAGR of 11.3 percent, according to a recently released report from EvaluatePharma.
In the United States, a rare disease is defined as a medical condition affecting fewer than 200,000 patients. Most are genetic, appearing early in life.
Examples of rare diseases include cystic fibrosis, glioma, pancreatic cancer, acute myeloid leukemia, multiple myeloma, renal cell carcinoma, ovarian cancer and Duchenne muscular dystrophy
Approximately 25-30 million Americans live with a rare disease, according to the National Center for Advancing Translation Sciences Genetic and Rare Disease Information Center (GARD). More than 7,000 rare diseases have been identified and 95 percent of these are without approved therapies.
Because the markets for these drugs are small, in many cases, pharmaceutical companies involved in this kind of drug discovery are eligible for financial incentives available from the government. The Orphan Drug Act of 1983 encourages the development of orphan drugs in the United States, offering a seven-year window of tax reductions and the exclusive right to market a drug for a particular rare disease. Similar legislation can be found in Europe, Japan, Singapore and Australia to encourage the development of drugs to treat orphan diseases.
Since the passage of the Orphan Drug Act of 1983, the number of orphan drugs coming to market has skyrocketed. There was just one drug with orphan designation in 1983, jumping to 40 the next year. The number of orphan drugs on the market reached 121 in the year 2007. Today, over 600 orphan drugs have received U.S. Food and Drug Administration (FDA) approval.
North America currently leads the world in orphan drug market growth, followed by Europe, Asia Pacific and the rest of the world, according to MarketWatch.
Orphan drugs will make up 21.7 percent of prescription sales in the year 2024, up from 17 percent today, the report estimates. The projected growth for 2024 is double the rate forecast for the non-orphan drug market.
Key players in this market—occupying nearly 50 percent of the orphan drug market—are Novartis AG, F. Hoffmann-La Roche Ltd, Celgene, Bristol-Myers Squibb Company, Shire pharmaceuticals, Pfizer, Sonafi and Bayer Healthcare, MarketWatch says.




