Every year, companies are coming out with new, innovative drug delivery technologies, improving ease of access and design by modifying the devices for easy administration and the best possible drug absorption.
3M Drug Delivery Systems, a part of the Health Care Business Group of 3M, researches and develops new ways to deliver drugs to patients and (ultimately) manufactures those devices. The company offers a wide array of drug delivery technologies—inhalation, nasal, oral, topical, transdermal, and more.
“Our process begins with identifying the needs of both patients and drug manufacturers,” said Ethan Trepp, Global Marketing Manager, 3M Drug Delivery Systems Division. “Because we know each illness and drug treatment is unique, we partner with drug manufacturers to develop the best approach to address those unique challenges.”
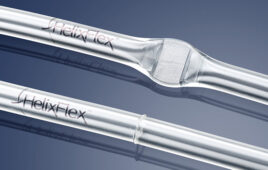
One of the company’s newest drug delivery devices is its microneedle technologies. 3M™ Hollow Microstructured Transdermal System (hMTS) delivers the vaccine directly to the patient’s dermis.
“It gives rise to a potentially stronger immune response, compared to traditional intramuscular injections,” said Trepp. “In other words, the intradermal delivery may enhance the efficacy of the drug. The hMTS system also enables consistent, reproducible delivery of the drug.”
It takes approximately one minute per milliliter to administer, up to 2.0 milliliters over a range of viscosities. For example, a 0.5 milliliter volume takes about 30 seconds.
“Shallow deliveries, such as to the dermis, are often challenging,” said Trepp, adding that microneedle technologies “could also improve patient adherence because the patient doesn’t necessarily have to go to a clinic to receive the drug. It could be administered at home by patients themselves or by a family member.”
To administer a drug using 3M’s microneedle technology, the process is to place the applicator on the skin and then press toward the skin anywhere on the applicator.
In addition to a change in administration, the storage is different from a traditional vaccine. A drug is applied to the tips of solid microneedles, which are then dried and sometimes refrigerated.
“Unlike standard vaccine delivery methods, which require the vaccine to be stored in a narrow, cold temperature range, this method can withstand a wider temperature range as the vaccine is transported from the location of manufacture to the patient. When we’re talking about reaching patients in third-world countries, for instance, reducing and eliminating the cold chain is a critical issue,” Trepp explained.
There also seems to be a potential use of microneedles for patients who are afraid of needles.
“Both solid and hollow microneedles can be very helpful for people with fear of needles. Studies show that fear causes roughly 10% of the population to avoid injectable drugs, leaving them vulnerable. Rather than one long needle that is injected into the muscle, microneedle technologies utilize many shorter needles that are applied to the skin surface layers, potentially reducing the level of pain,” said Trepp.
Not everyone will necessarily benefit from this administration method, however. Specifically, solid microneedles have a dose level limitation. Trepp explained that “only a certain amount of drug, hundreds of micrograms, can be coated and still delivered efficiently in a single dose.”
3M’s microneedle technology, hMTS, is currently in clinical trials and not yet approved for patient use.
“Only certain drugs are available using this technology,” said Trepp. “A drug and the device through which it is delivered are approved together by the FDA as one system. They are not approved independently of one another.”
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!