
Image from Fernando Zhiminaicela on Pixabay
While vaccine hesitancy will be a barrier in battling the COVID-19 pandemic, the storage requirements for SARS-CoV-2 vaccines will invariably be another significant hurdle.
The BNT162b2 vaccine candidate from Pfizer (NYSE:PFE) — with its subarctic storage requirements of -70°C — could be especially vexing. Approximately 5% of the vaccine, which Pfizer developed with partner BioNTech (NSDQ:BNTX), could be lost in transit as a result of temperature excursions, according to Jean-Pierre Emond, CEO of BlueEye LLC, who was quoted in a UBS briefing note.
Assuming the vaccine wins an FDA EUA soon, approximately 6.4 million doses of the COVID-19 vaccine could ship to U.S. states in mid-December.
Patients receiving the vaccine must receive two doses 21 days apart.
Pfizer has worked to simplify the challenge of safely distributing the vaccine by developing suitcase-sized dry-ice-cooled storage containers for the vaccine. The containers, however, can only be opened twice each day for less than three minutes each time. Exceeding those requirements can cause the vaccine temperature to warm beyond the recommended specifications.
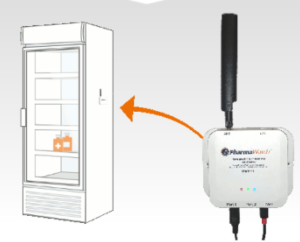
PharmaWatch’s technology uses LTE Cat-M1 cellular technology to transmit temperature data.
Temperature excursions are also possible after COVID-19 vaccines reach their final destination. Few healthcare facilities and retail pharmacies have experience with ultracold storage or ultracold temperature monitoring.
“Traditional monitoring techniques that use data loggers or mercury thermometers are not going to work in those ultra-cold environments,” said Nicholas Ioli, chief operating officer of PharmaWatch, a company offering continuous temperature monitoring for healthcare environments, including those planning on storing COVID-19 vaccines.
Even for vaccine candidates with less-stringent requirements, temperature excursions should be a worry.
“If the cold chain is not properly maintained, vaccine potency may be lost, resulting in a useless vaccine supply,” warned the Centers for Disease Control and Prevention (CDC). Temperature-compromised vaccines could also be responsible for adverse events, although data on that subject is lacking.
“With COVID vaccines, there hasn’t been a lot of runtime on stability studies,” Ioli said. “There’s not a lot of data to support how stable or resilient these vaccines are to conditions that would compromise their temperature.”
Many healthcare facilities and retail pharmacies lack the resolution to safeguard COVID-19 vaccines’ potency, according to Lindsay Meloy, sales and account executive at PharmaWatch. CDC recommends using a digital data logger that records at intervals of at least every 30 minutes. But organizations storing vaccines “really need a continuous temperature monitoring system,” Meloy said.
PharmaWatch’s technology offers a five-minute sampling rate and continuous monitoring to ensure that the vaccines are not compromised by temperature excursions.
They also need a functional alerting system, she added. If a freezer goes “out of range in the middle of the night, somebody should get an alert so they can deal with that before the vaccine’s viability is affected,” Meloy added.
PharmaWatch did a study with the state of Arkansas, which implemented real-time vaccine monitoring. State officials documented more than 300 instances of temperature excursions over a five-year period that would have been undetected with traditional temperature logging.
Another wrinkle is that smaller hospitals in the U.S. and developing countries across the world can ill afford ultracold freezers, which cost in the range of $10,000 to $15,000. According to the University of Michigan, the electrical costs of operating such freezers can range from $750 to $1000 per year.
The CDC is not currently recommending that state health authorities purchase such ultracold units. The agency expects vaccines with less-stringent storage requirements to be available soon.
Demand for such freezers, however, has exploded in recent months.
Dry ice can also keep the vaccines cold, but the low-tech method of cooling has disadvantages. “Dry ice is finite,” Ioli said.” “It only lasts so long. And, you know, as it sublimates, you get temperatures that start to increase, and at what point is too much for vaccine stability?”
Dry ice producers also worry that a spike in demand next year could lead to shortages. Demand for dry ice, which is made by injecting liquefied carbon dioxide into a holding tank to be frozen, has surged this year, along with demand for home grocery deliveries. Carbon-dioxide, however, is a coproduct of ethanol, whose production has fallen in 2020.
Thankfully, there has been a growing awareness of the need for effective temperature management of vaccines in transit or storage. “Pharmaceutical companies are engaging with cold-storage specialists to ensure a smooth supply of the vaccine when it does become commercially available,” said Vinie Varkey, a senior pharma analyst at GlobalData.
Healthcare workers also seem to be increasingly aware of the topic, Ioli said. “I’ve spoken with nurses, physicians and pharmacists who have said: ‘I’ll only get the COVID-19 vaccine from facilities that are monitored in real-time, and where I can make sure that their cold chain was clean from manufacturer to the point of use.’”
Next year, Pfizer plans on introducing a powdered version of the vaccine, which would be stable at higher temperatures.





Tell Us What You Think!