Image from Hyttalo Souza on Unsplash
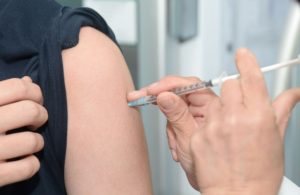
Some 72 people have died in South Korea after receiving flu shots, leading several physicians to call for a pause in immunizations. Government officials initially reported there was no clear link between the deaths and the vaccines. Among the casualties was a 17-year-old high school student.
The Korea Disease Control and Prevention Agency (KDCA) decided the deaths were unrelated to its free flu inoculation program, and vowed to press forward with it.
To make its determination, KDCA reviewed autopsies of 71 patients, most of whom were elderly and had prior health problems. The cause of the remaining death is unknown, according to Newsweek.
When the tally of deaths stood at 25, KDCA Commissioner Jeong Eun-kyeong said there was a low possibility the vaccine was to blame, according to Reuters.
Many health officials across the world stress that flu vaccines are safe for the vast majority of the population. Singapore, however, temporarily stopped using two flu vaccines following the string of deaths in South Korea, according to Reuters. No deaths linked to influenza vaccines have been reported in Singapore.
Before South Korea’s 2020 vaccine program began, KDCA temporarily stopped the program as a result of logistical problems. A vaccine batch was inadvertently stored at room temperature, prompting a recall. Roughly 2,300 doses had already been administered, according to the New York Times. The mishap would cause the flu vaccine to lose potency but would likely not make it toxic.
In a separate event, a flu vaccine provider initiated a recall involving 615,000 doses after white particles were discovered in some of the vials. Nearly 18,000 people received the doses before the recall.
The Korean Medical Association had called for an investigation into the flu vaccination program.
This story originally ran on October 23. Updated on October 30.





Tell Us What You Think!