Photo via: https://www.fda.gov/Drugs/DrugSafety/ucm570237.htm
Balguti Kesaria Ayurvedic Medicine: FDA Warning – High Levels Of Lead
ISSUE:
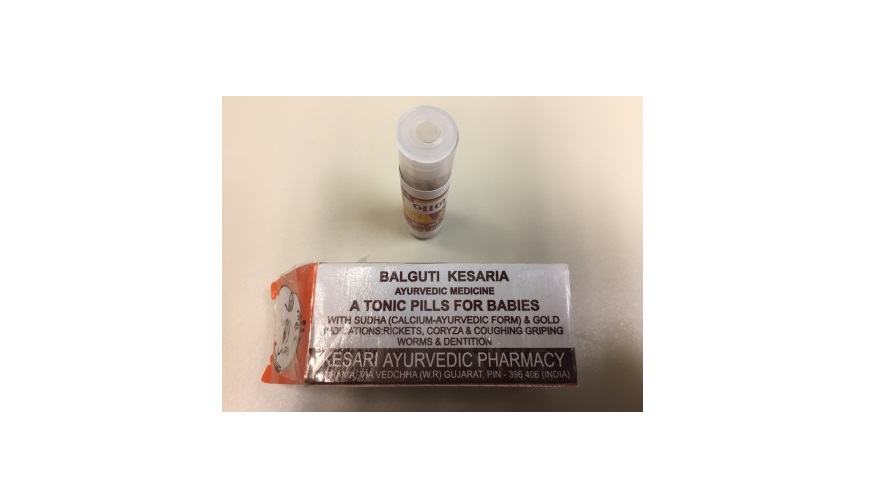
The U.S. Food and Drug Administration is warning parents and caregivers not to use “Balguti Kesaria (or Kesaria Balguti) Ayurvedic Medicine” due to the risk of lead poisoning.
FDA has not reviewed this product for safety or effectiveness. Exposure to lead can cause serious damage to the central nervous system, the kidneys and the immune system. In children, chronic exposure to lead—even at low levels—is associated with impaired cognitive function, including reduced IQ, behavioral difficulties, and other problems.
BACKGROUND:
This product is sold online and manufactured by multiple companies, including Kesari Ayurvedic Pharmacy in India. Individuals have also mailed or brought the product into the United States. “Balguti Kesaria Ayurvedic Medicine” is used with infants and children for a variety of conditions including rickets, cough and cold, worms and dentition (teething).
FDA initially learned of this risk from the North Carolina Division of Public Health after the product was tested and found to contain high levels of lead. FDA was also notified by the Michigan Department of Health and Human Services of high levels of lead in two children who were given this product. Michigan’s testing also found high levels of lead in the product. To date, FDA has received one adverse event report of high levels of lead and developmental delays in a child who was given this product.
RECOMMENDATION:
Anyone who is using this product or giving it to a child should stop immediately and consult a health care professional.
(Source: FDA)
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




