(Credit: PRNewsFoto/Galderma)
Galderma announces FDA approval of Restylane Refyne and Restylane Defyne dermal fillers for treatment of ‘laugh lines.’
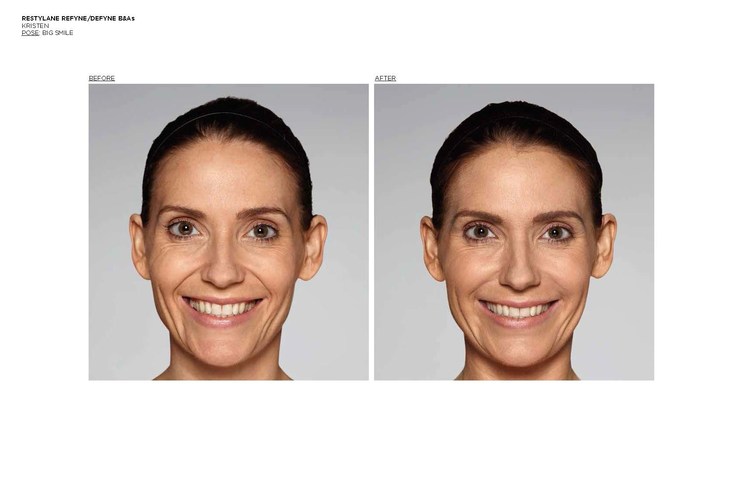
Galderma, a global company focused on medical solutions in skin health, announced it has received U.S. Food and Drug Administration (FDA) approval of two new products for the treatment of nasolabial folds (NLF) or “laugh lines,” in patients over the age of 21.1,2
Restylane Refyne was approved for the treatment of moderate to severe facial wrinkles and folds and Restylane Defyne for the treatment of moderate to severe, deep facial wrinkles and folds.1,2
The scientifically-advanced gels are manufactured with XpresHAn Technology, creating gels that offer a range of flexibility and support3,4,5 for varied patient needs. Restylane Refyne and Restylane Defyne have been shown to maintain effectiveness for the treatment of laugh lines for up to 12 months.1,2
“Even with current approved options, many of my patients are still looking for different solutions to treat their laugh lines,” said Boca Raton-based oculoplastic surgeon and Restylane Refyne clinical investigator Steven Fagien, M.D. “These new products are flexible and are designed to meet different patient needs. I am excited to offer these next-generation hyaluronic acid (HA) dermal fillers in my practice.”
“Restylane Refyne and Restylane Defyne are the latest FDA-approved advancements in HA dermal fillers and align with Galderma’s mission to help individuals achieve natural-looking results through treatments with a long-standing history of proven safety and efficacy,” said Kelly Huang, Ph.D., VP & general manager of the U.S. Aesthetic and Corrective business of Galderma. “We saw an opportunity to address a common concern for patients who have not yet tried a dermal filler by designing gels that provide natural-looking results. With these new brands, the Restylane family of products now represents the broadest offering of HA dermal fillers in the U.S.”
The FDA approval was based on two pivotal, double-blinded, randomized, active-controlled Phase 3 studies investigating Restylane Refyne and Restylane Defyne (involving 171 and 162 subjects, respectively) to evaluate their safety and effectiveness. In both studies, Restylane Refyne and Restylane Defyne met the studies’ endpoints, with both products showing a clinically meaningful improvement in wrinkle severity for up to 12 months in the majority of patients.
Study investigators used the Wrinkle Severity Rating Scale (WSRS), a validated 5-point measure of the size and depth of the wrinkles, with grade 1 defined as absence of wrinkles and grade 5 as extremely deep and long wrinkles. Investigators reported that 79% of Restylane Refyne subjects and 77% of Restylane Defyne subjects had at least a 1-grade improvement on the WSRS after 6 weeks.
Subjects also performed self-assessments (SSA) of wrinkle severity, with most subjects reporting at least a 1-grade improvement in SSA scores with Restylane Refyne and with Restylane Defyne after 6 weeks.1,2
“Many of my patients are interested to learn about the latest products that can help them achieve natural-looking results, but are oftentimes unsure about starting dermal fillers,” said San Diego-based board-certified dermatologist Mitch Goldman, M.D. “The introduction of these next-generation HA dermal fillers with XpresHAn Technology has the potential to change my patients’ views on fillers. Restylane Refyne and Restylane Defyne provide options for patients who want to make sure they can achieve natural-looking results, which is a key need my patients express every day.”
After initial treatment, injection site responses (redness, swelling, bruising, lump/bump formation, pain/tenderness) were predominantly mild or moderate in intensity, temporary (typically with a duration of one to two weeks), and similar for the Restylane products.
__________________________________________________
References:
1 Galderma. Restylane Refyne Instructions for Use. 2016.
2 Galderma. Restylane Defyne Instructions for Use. 2016.
3 A pivotal USA randomized evaluator-blinded, active-controlled, multi-center, split-face comparison study of Emervel Deep Lidocaine versus Juvederm Ultra Plus in the treatment of moderate to severe facial wrinkles and folds [Phase 3 Support Study: RD.06.CIR.18159 CSR Emervel Deep, phase 3 vs Juvederm Ultra Plus, 79w]
4 A pivotal USA randomized evaluator-blinded, active-controlled, multi-center, split-face comparison study of Emervel Classic [Phase 3 Support Study: RD.06.CIR.18156 CSR Emervel Classic, phase 3 vs Juvederm, 76w]
Lidocaine versus Juvederm Ultra in the treatment of moderate to severe facial wrinkles and folds
5 Evaluation of amplitude sweep cross-over point as an index of flexibility [MA-32418 Final Flexibility Report], October 4, 2016.
(Source: PR Newswire)




