As of June 30, 2017, the U.S. FDA extended the Product Identifier Requirements under the Drug Supply Chain Security Act (DSCSA).
The previous implementation timeline dictated that all manufacturers must have serialized products after November 2017.
According to the new Guidance document, titled Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance Policy Guidance for Industry:
FDA does not intend to take action against manufacturers who do not, prior to November 27, 2018, affix or imprint a product identifier to each package and homogenous case of product intended to be introduced in a transaction into commerce as required by section 582(b)(2)(A) of the FD&C Act.
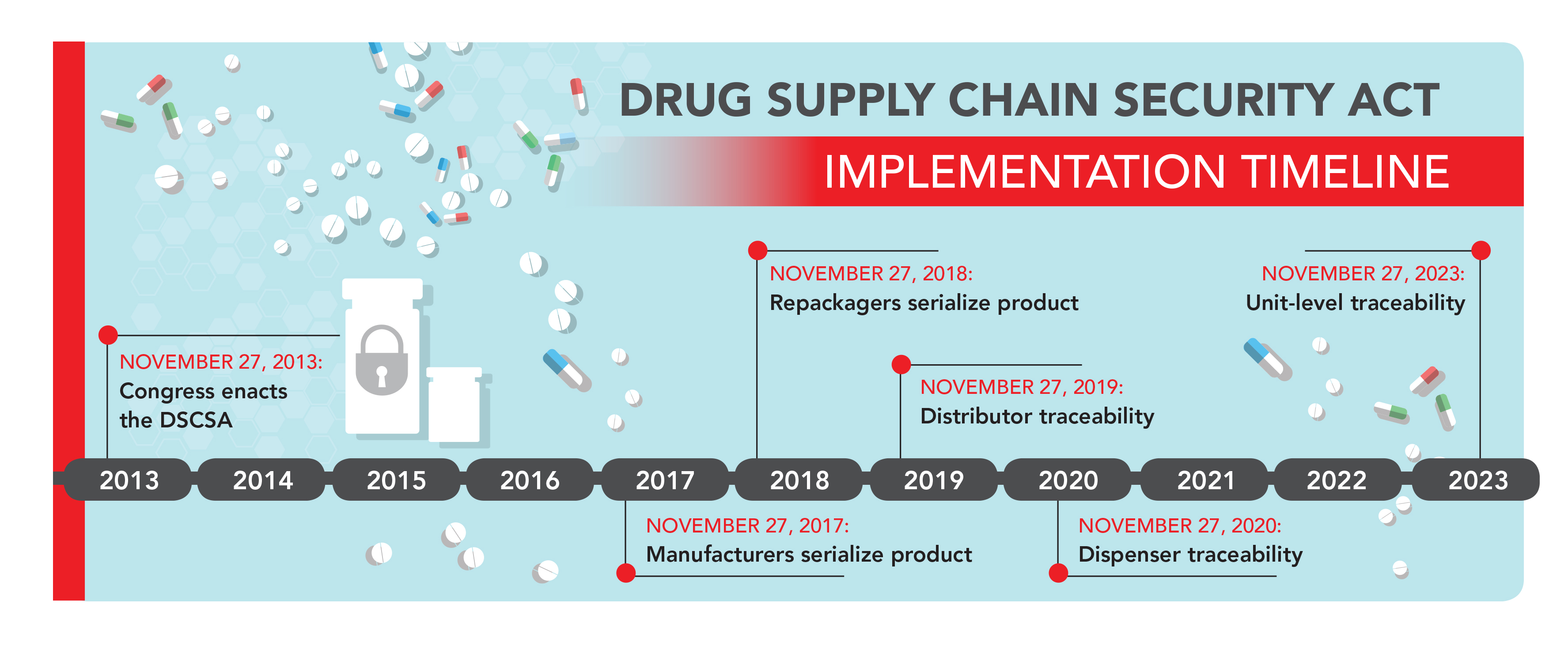
What does that mean for pharmaceutical manufacturers? In short, companies won’t be penalized if they don’t meet the November 27, 2017 serialization deadline.
The FDA’s announcement of the Draft Guidance further goes on to say:
This guidance informs manufacturers and other supply chain stakeholders that although manufacturers are to begin including a product identifier on prescription drug packages and cases on November 27, 2017, the FDA is delaying enforcement of those requirements until November 2018 to provide manufacturers additional time and avoid supply disruptions.
Several industry experts have commented on what this change means for the industry.
Brian Daleiden, Vice President Industry Marketing, TraceLink, comments:
With the recent announcement by the FDA of a delay in the active enforcement of the initial Drug Supply Chain Security Act (DSCSA) serialization requirements for pharmaceutical manufacturers, companies now have a little more time to implement their serialization programs correctly and efficiently. While not a delay in the law itself, this enforcement discretion will help to ensure that companies are not unduly penalized for failing to meet the deadlines. The time and effort for a company and its supply ecosystem to implement a serialization solution is often under-estimated, as are the impacts to business operations. We have seen that many companies, in particular mid-sized and small companies that use contract manufacturing organizations (CMOs), are only just realizing the complexities of the task at hand. In fact, several surveys carried out by TraceLink and others have shown that lack of readiness in the external supply ecosystem with CMOs is a contributing factor as to why pharmaceutical manufacturers are not ready for the 2017 DSCSA deadlines. Now is the time to continue pushing ahead, not to pull back. Acting now will ensure that project resources stay focused, accumulated serialization knowledge is utilized appropriately, relationships with supply partners are maintained, and issues are identified early on to minimize disruption.
Dexter Tjoa, Director Corporate Strategy, Tjoapack, comments:
It is important to note that the DSCSA regulations do remain in effect and the serialization requirements for manufacturers still become effective on November 27, 2017. The FDA only intends not to act against non-compliance. In practice, I expect this means that they will not actively probe for non-compliance and will judge any non-compliance incident on a case-by-case basis. However, the FDA can still enforce the regulations and associated penalties from November 27 onwards.
I think it is important that manufacturers and CMOs/CPOs do not become complacent and continue their serialization projects as originally planned. It must be remembered that an average serialization implementation program takes roughly 18 months—so for those who haven’t started yet, it is not guaranteed they will be ready for the November 2018 enforcement date either. Serialization implementation remains a complex task that doesn’t stop when you reach compliance. Incorporating serialization as a business-as-usual process and optimizing the new serialized packaging processes will take time beyond the original project.
However, if a manufacturer does want to revise their planning, they should make sure that their internal and external manufacturing network has the capacity to accommodate the new planning to avoid competition with implementation projects for other serialization regulations, such as the EU FMD.
Carlos Machado, Serialization Director, SEA Vision U.S., comments:
Serialization is a complex process that requires effective planning, yet many pharmaceutical manufacturers appear to have under-estimated the time taken to fully implement a solution, with some smaller and mid-sized companies yet to make a start. A number of serialization suppliers are also experiencing challenges and delays during projects due to ineffective planning which is creating bottlenecks in the supply chain and impacting the quality and service that they can provide. The FDA response to the concerns over industry wide readiness highlights the need for companies to act now, although active enforcement won’t take place until November 2018. Over half of respondents in our recent industry research said that they are not currently preparing for the upcoming regulations because they lack sufficient internal resource to devote to serialization. The delay in the active enforcement of the Drug Supply Chain Security Act (DSCSA) provides companies who are lagging behind with additional time to identify reputable implementation partners, who deliver on time and on budget, and with a track record of success that can help oversee the successful rollout of serialization projects.
Staffan Widengren, Director Corporate Projects, Recipharm, comments:
The DSCSA enforcement delay is very much a result of companies underestimating the scale and complexity of the serialization challenge. Recipharm recognized the enormity of the task of preparing for serialization at an early stage and established a taskforce dedicated to ensuring compliance in time for both the U.S. and European deadlines. It is important that companies preparing for the EU Falsified Medicines Directive (FMD), due to be enforced in February 2019, learn from the complacency that has led to this delay and act now to ensure that a robust solution is in place. They must commit time to understanding both the market and technology requirements, engage with the necessary third party expertise to implement an effective solution and ensure continuity of supply, and focus on building a process that is scalable and flexible enough to adapt to changing regulatory and business demands.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




