Exosomics Siena S.p.A., a Lonza-backed diagnostics company, has launched its first proprietary liquid biopsy technologies, SeleCTEV DNA and SortEV RNA, for the selective isolation of tumor-derived nucleic acids from complex biofluids, such as plasma or serum.
An Italian start-up company, Exosomics Siena develops exosome-based early-stage cancer screening and molecular diagnostic tests. In May 2017, Lonza invested in Exosomics Siena to help to support the continued research, development, and pre-commercialization of the non-invasive tests.
Distinct from current liquid biopsy methods, where total nucleic acids are harvested from blood with low amounts of tumor-derived genetic material, Exosomics Siena’s technologies can provide pre-analytical kits based on enriched tumor-derived exosomes and therefore enriched tumor-derived genetic material.
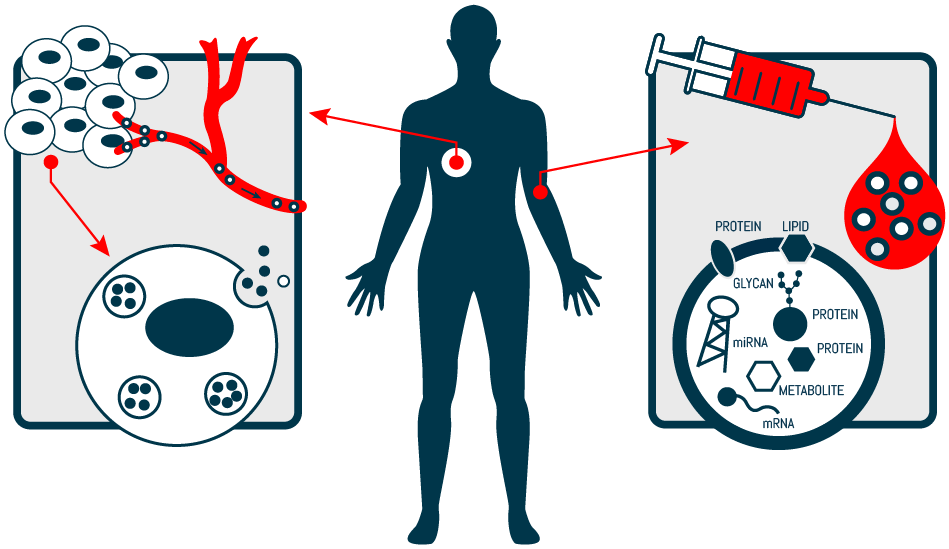
Next generation technology: A world in which cancer diagnosis is possible from a simple blood draw. (Credit: Exosomics Siena S.p.A. via www.exosomics.it/technology)
Exosomes are small extracellular vesicles present in all human biofluids and contain DNA, RNA, and proteins originating from their parent cells. Enriching tumor exosomes, based on relevant pan-tumor markers, offers a more specific and sensitive diagnostic approach when compared with traditional liquid biopsy methods.
SeleCTEV DNA and SortEV RNA kits use proprietary technology to purify tumor-derived exosomes. Each technology employs a unique affinity method to select tumor-derived exosomes from which the DNA or RNA, respectively, can be extracted. Additionally, the SeleCTEV DNA kit can be used to isolate circulating tumor-derived, cell-free DNA in addition to exosome-shuttled DNA.
At this time, the kits are released for research use only.
Exosomics Siena said it also is working on the development and clinical validation of an early pan-cancer screening assay projected to be launched in 2021. The Cancer-rEVeal assay will demonstrate both the power of exosomes as a vehicle of multiple classes of biomarkers relevant for next-generation diagnostics.
“As part of Lonza’s wider strategy in developing exosomes, we have chosen to invest in Exosomics Siena as we believe their approach will have advantages over other non-invasive cancer tests currently on the market or in development,” Behzad Mahdavi, Ph.D., MBA, chairman of the board for Exosomics Siena and vice president, strategic innovation at Lonza, said.”
Lonza last year acquired HansaBioMed Life Sciences, Tallinn, Estonia, a start-up company dedicated to the research and development, manufacturing, and distribution of products for the exosomes research market.
Founded in 1897 and headquartered in Basel, Switzerland, Lonza is a global biopharmaceutical development and manufacturing company with more than 100 sites and offices and approximately 14,500 full-time employees worldwide.




