An exclusive roundtable with Cambrex and West Pharmaceutical Services, Inc. on contract manufacturing as a whole, how the space has changed, upcoming trends, as well as what the industry will look like in the coming years.
2016 saw a continued increase in outsourcing manufacturing operations to contract manufacturers in order for pharmaceutical companies to cut back on in-house costs and capacity while maintaining quality and improving time to market. With increased pressures for companies to reduce cost while still expanding core competencies (such as R&D) and marketing, outsourcing continues to make a name for itself in the industry.
A number of contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) have expanded their offerings to meet market demands, becoming even more versatile as the demand for a greater availability of drugs and swifter drug approvals increases (with more regulations in place).
Meanwhile, some contract manufacturers have found their niche in the market and continue to make a name for themselves within that space.

(Credit: Cambrex)
CMO Cambrex focuses solely upon small molecule chemical development and manufacturing. With ambitions to become the number one small molecule CMO in the pharmaceutical industry, Cambrex has recently facilitated the building of a $50-million, 7,500-square foot manufacturing plant in Charles City, IA and intend to meet their growing customers demand while expanding operations. The company has manufacturing facilities in Sweden and Italy as well.
West Pharmaceutical Services, Inc. offers customized contract manufacturing solutions that address market needs. Formerly known as The Tech Group, West’s CMO business has been in operation for 50 years (though, West officially purchased The Tech Group in 2005). When founded, the company worked primarily in building precision injection molds and molding product in the aerospace and avionic industry. Today the company specializes in the assembly of drug delivery systems, combination products, and medical devices—injection molding specifically—and is a supplier to the pharmaceutical, medical and consumer industries.
Representatives from both of these contract manufacturers participated in a roundtable with Pharmaceutical Processing on contract manufacturing as a whole, how the space has changed, upcoming trends, as well as what the industry will look like in the coming years. Shawn P. Cavanagh, Executive Vice President and Chief Operating Officer, spoke on behalf of Cambrex, and Mike Treadaway, Vice President, General Manager, Contract Manufacturing, spoke on behalf of West’s Contract Manufacturing team (formerly known as The Tech Group). Their edited responses are below.

(Credit: West Pharmaceutical Services, Inc.)
Q: What are some of the trends you’re seeing in the contract manufacturing space?
Cavanagh of Cambrex:
There is continued worldwide growth in API consumption and increased outsourcing requirements by large innovator companies. Coupled with the increasing use of generics, with additional opportunities for further penetration in developing markets, we expect the industry to continue to experience strong growth. The growth over the past few years has resulted in some capacity constraints in the industry, especially in the U.S.
Overall, growth in the contract development and manufacturing industry will see a high demand for suppliers with flexible large-, mid-, and small-scale cGMP capacity and world-class quality systems that allow efficient API production.
The industry has seen an increase in drug approvals in recent years and will continue to see the shift in pharmaceutical companies sourcing back to U.S. and European contract manufacturers after their less than positive experiences in low-cost countries in Asia.
Many larger pharmaceutical companies continue to divest their own production facilities and the rise of ‘virtual’ pharma has increased the demand for contract manufacturing organizations.
Today, the industry has the fastest growing small-molecule clinical pipeline reported in the last 20 years—with more small molecules in Phase I, II, and III than ever before. This solid pipeline of late-stage drugs will continue to drive the success of the fine chemicals industry.

(Credit: Cambrex)
Treadaway of West Pharmaceutical Services:
One of the biggest trends we’re seeing right now is a lot of consolidation. That has some pretty significant impact. We’ve seen a number of our competitors being acquired by major contract manufacturers, particularly in the electronics space.
Other trends that we’re seeing is contract manufacturers providing more and more services that traditionally would have been done by the OEM (or the pharmaceutical company).
If you look at our evolution as a company, we started out as a contract molder. Then, as our customers became more comfortable with us, we started doing some assembly work and then we started doing full assembly. Similarly, as OEMs became more comfortable, contract manufacturers added the quality systems and supply chain management systems to add more value. We evolved by adding that value over time. In some cases, we even assembled drug product in devices, which would’ve been unheard of 10 years ago.

(Credit: West Pharmaceutical Services, Inc.)
Q: How has the contract manufacturing space changed in the past five or 10 years? How is it similar?
Cavanagh of Cambrex:
Demand in the volume of small molecules has grown from ~160 thousand tons/yr in 2005 to ~325 thousand tons in 2016, while the number of FDA approvals for small molecule drugs has grown significantly from a lull witnessed in the 2005-7 period.
In previous years, we witnessed many new CMO entrants from China and India. This trend has slowed and demand for Western CMOs with the ‘right’ assets, technologies and capacities, rather than low-cost options, has now increased and many Western CMOs are now full compared to pre-2010 and, in fact, capacity in many cases is unavailable within 6 months or beyond.
Treadaway of West Pharmaceutical Services:
The pharma companies (OEMs) are more comfortable having the contract manufacturers provide more of those services that they were generally doing themselves.
In the early years, providing additional services was more opportunistic (for us). But we saw the industry moving that way and for that reason we started developing capabilities and adding processes that we could share with our customers and demonstrate how we could do that additional, value-added work.
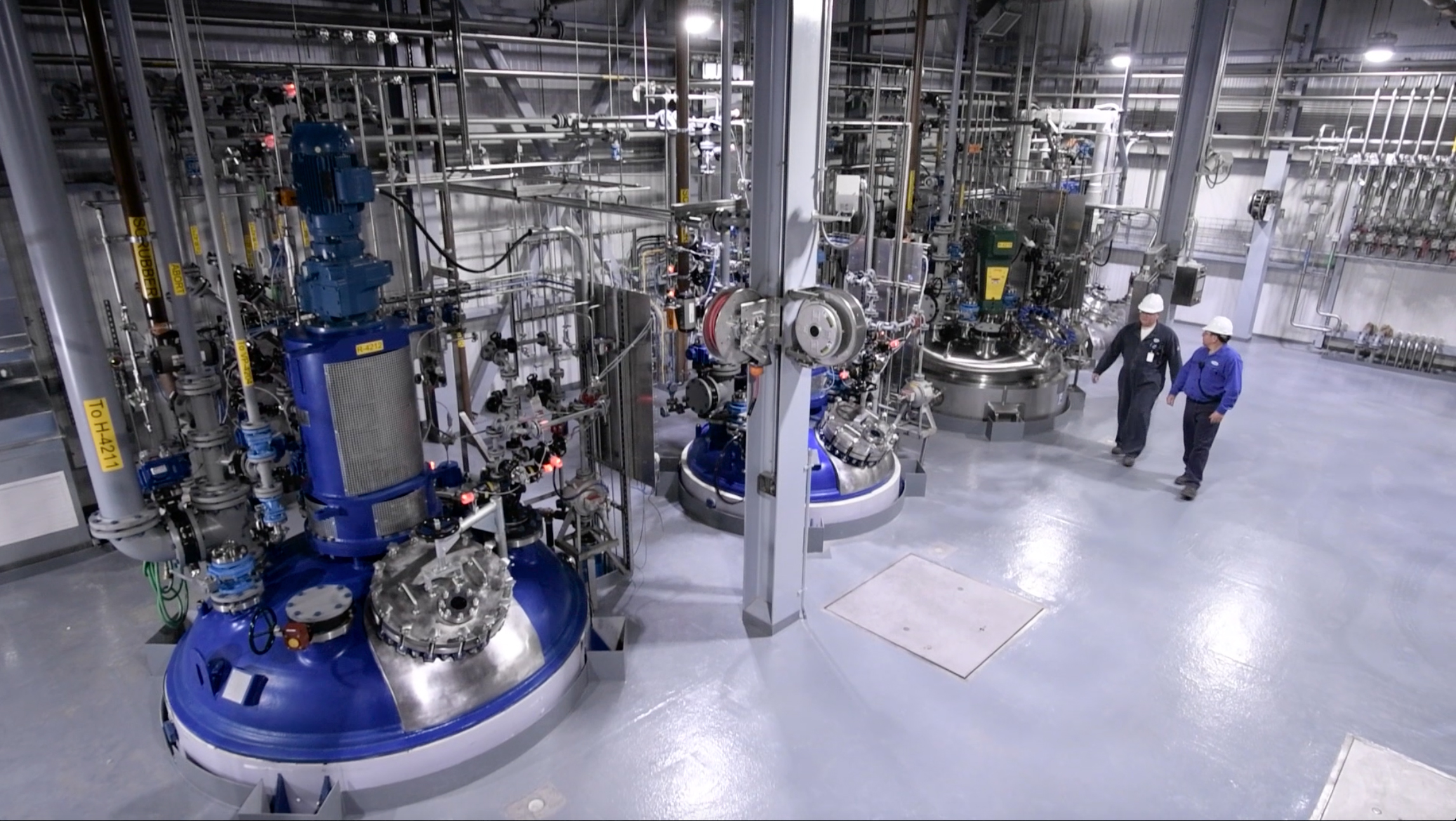
(Credit: Cambrex)
Q: How has the increased number of drug approvals impacted your operations?
Cavanagh of Cambrex:
There is reason for increased optimism due to a rebound in drug product approvals to levels seen in early 2000. This is due to successful focused R&D programs coming to fruition, as well as increasing numbers of specialty and orphan drugs and fast-tracked review processes.
In light of these recent drug approvals, targeting specialized therapies such as oncology or orphan diseases, the effect has been a rise in demand for small molecule manufacturing capacity in the 10s of metric ton range compared to the larger volumes and 100s of metric ton range seen decades before. This will ultimately lead to continued or increased outsourcing by large pharmaceutical companies, many with mature, over-sized captive capacity, to select CMO partners with the ‘right’ capacity for their project.
This will create significant opportunities for CMOs, in areas from raw materials to starting materials, registered starting materials, intermediates and advanced intermediates, as well as APIs.
Treadaway of West Pharmaceutical Services:
It’s really what’s fueling our growth—the number of drugs coming to market plus the trend for self-injection/self-administration. It is really impacting our operations.
We’re definitely seeing growth in biologics and the growth in the diabetes market is certainly impacting our business and opportunities for growth.
For self-injection, there are a lot of unique devices being developed that are specific to the market (and to the patient). That’s driving volume in injection systems.

(Credit: West Pharmaceutical Services, Inc.)
Q: Where do you see the biggest opportunity for growth in the CMO/CDMO market in the coming years?
Cavanagh of Cambrex:
Over the last decade, many Western pharmaceutical companies made a move to source APIs from low-cost countries such as India and China. Over recent years, we have seen (and continue to see) many pharmaceutical companies migrating back to Western suppliers for clinical products and increased API outsourcing to Western CMOs, purportedly for their higher quality, reliability, and delivery standards.
The market will continue to enjoy stable growth, particularly for reliable U.S. and European suppliers such as Cambrex that can meet the increasing demand for outsourcing of APIs and intermediates.
Treadaway of West Pharmaceutical Services:
For us, it’s connected health. We’re seeing a real convergence—particularly because we do a lot of work in diagnostics and drug delivery systems. We’re seeing electronics come into play where drug delivery systems have on-board capability for communicating when a dose was given, how much of a dose was given, connecting to a diagnostics device. That whole connected health piece is a huge opportunity for the future.
The other area is emerging markets. As healthcare becomes more available, we’re certainly seeing a lot of opportunity for drug delivery systems and diagnostic products in some of these markets.
This cover story can also be found in the March 2017 issue of Pharmaceutical Processing.
Feature image credit: West Pharmaceutical Services, Inc.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




