In an effort to make drug development and clinical trials more efficient, life sciences companies and regulatory agencies are demanding higher levels of coordination in trial master file (TMF) processes. Regulatory agencies such as the European Medicines Agency (EMA) and Medicines & Healthcare products Regulatory Agency (MHRA), for example, minimally expect that a company’s TMF accurately reconstruct how a clinical trial was conducted to demonstrate effective sponsor oversight, support decisions made, and comply with Good Clinical Practices (GCP) guidelines.
Despite the technology options available today, many life sciences organizations struggle to meet this minimum expectation. The MHRA says that 35 percent of site inspections from 2014 were delayed because of incomplete or unavailable TMFs.1 Part of this has to do with a lack of purpose-built technology solutions, but it is also a result of not adjusting processes to fully leverage modern technology. So, even as the industry shifts away from manual, paper-based systems, it struggles to shake familiar, paper-based processes.
According to the annual Veeva 2015 Paperless TMF Survey*, which explored the life sciences industry’s progress toward paperless clinical trials by gathering data from TMF owners around the globe, most TMF processes remain rooted in paper. More than half of the respondents (59 percent) now archive documents electronically, but few have digitized key processes such as e-signatures (21 percent), document creation (25 percent), and collaboration (30 percent).2
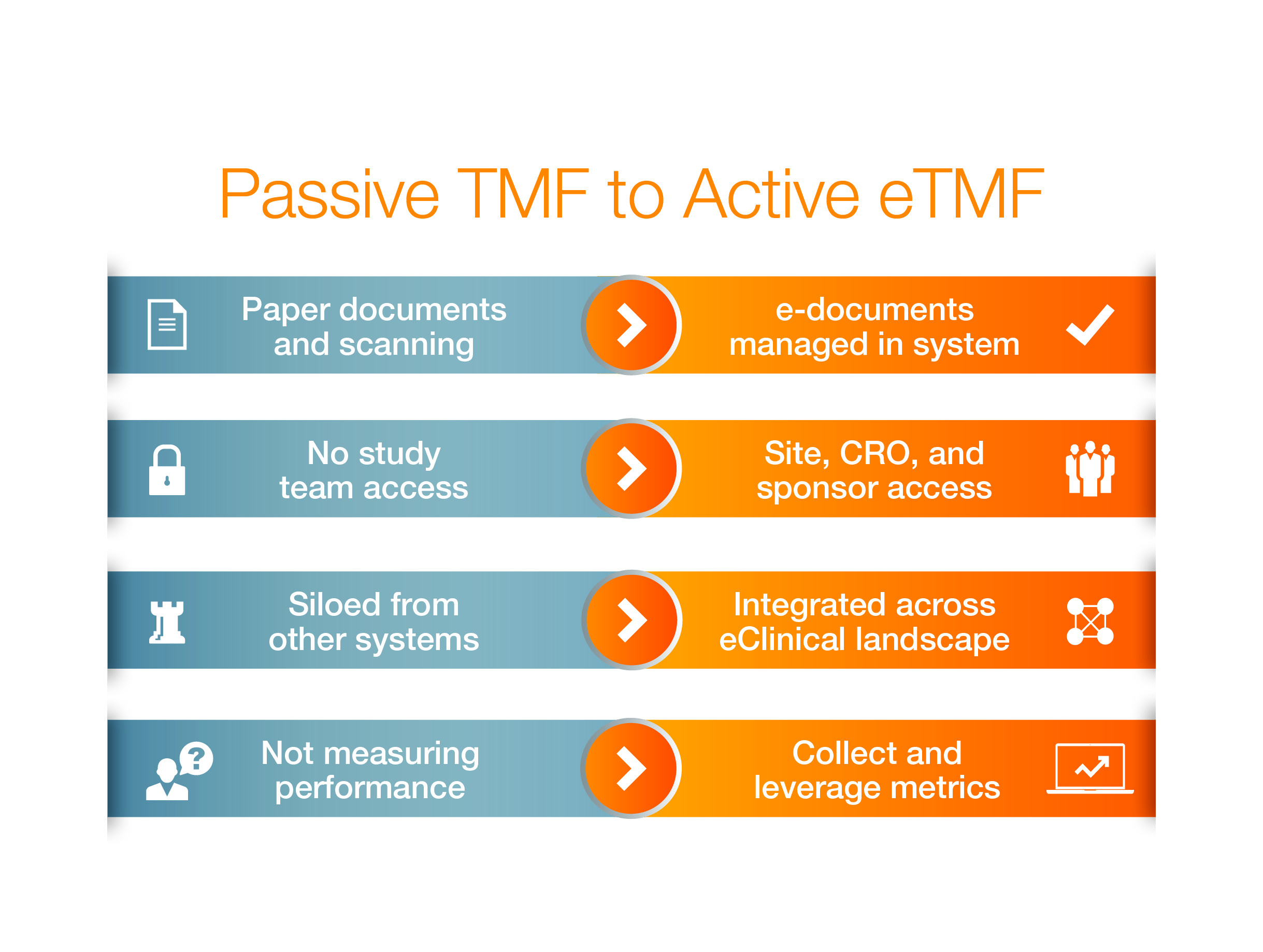
Technology has automated some aspects of the clinical trial process, but TMF operating models remain little more than electronic replica of paper methods. To put it another way, old habits die hard. Life sciences companies must rethink not only their technology platform, but also their approach to TMF management. By shifting to a more efficient, active TMF model, companies will be positioned to thrive amidst today’s regulatory environment, competitive global marketplace, and increasingly complex partner network.
Unfortunately, most companies are stuck in a passive TMF model where data and documents are archived at the end of a trial rather than in real time, as part of the natural workflow. Conversely, an active model for TMF management ensures all TMF documents, data, and processes are managed in the system as they are being executed. Let’s look at both approaches more closely.
Passive vs. Active: Winner Takes All
In a passive TMF operating model, documents are uploaded into the system only after they are sent, scanned, and finalized. The underlying, inefficient paper processes of yesterday persist, only now documents are in an electronic format. Upfront work is still conducted outside the system, documents are stored in multiple locations, and content is not tracked in progress—all of which keeps TMF activity segregated from broader clinical processes. This makes it difficult to see study status at all times and capture valuable metrics, all of which are used to evaluate TMF compliance and to reconstruct how trials were run.
Additionally, passive TMF management leads to multiple versions of documents, sluggish workflows and delays, and futile metrics. In fact, it is impossible to use metrics to influence business decisions or as a catalyst for improvement with a passive approach since only the end results are measured. Companies cannot respond throughout the course of a study, make changes, or re-direct resources.
“A passive TMF model promotes inefficiency and waste that can trickle down to broader clinical trial processes,” explained Jessica Vicari, director of regulatory start-up and document management at Advanced Clinical, a full-service contract research organization (CRO). “When all of the sponsor’s functional teams and external partners share central TMF access to execute trial-driving business processes, there is enormously valuable data to analyze and act upon. An active eTMF is not only an engine aggregating meaningful metrics in real time, but also an effective means for streamlined collaboration. Whereas when study documents reside in multiple, disparate systems, it takes a massive effort to collect and assess them, and even then it’s likely the end result will be incomplete. Worse still, life sciences companies struggle to collaborate efficiently with all parties.”
Shifting to an active eTMF model is a more effective way to reconstruct how a trial is run because the TMF is maintained in a constant state of inspection readiness. TMF composition is an automatic result of an executed clinical process. Therefore, compliance is continuous and operational metrics are easy to collect and monitor in real time.
For example, documents are created, reviewed, and accessed by all study partners in one system. With a single source of truth for all TMF documents and information, there are no version control issues or time-consuming document reconciliation at study close out. Automated workflows replace outdated paper-based, iterative processes, and ensure TMF quality and timeliness.
Active TMF management is a relatively new concept and is a direct result of the evolution of technology supporting TMF operations. Systems and processes are indistinguishable in this model because they work in tandem to reach the end state. Traditional content management systems and collaboration tools are incapable of supporting the complex workflows or providing the access to data, documents, and processes necessary to enable active TMF management. Applications designed specifically for TMF processes provide access for all stakeholders and enable the collection of valuable metrics to ensure TMF completeness and accuracy.
In contrast to the manual reporting consistent with a passive TMF approach, an active model makes study progress visible to all stakeholders throughout the trial, enabling faster response to issues. Enterprise-wide information is available for organizational analysis and continuous improvement too, as opposed to quality-control checks after each step of the trial, followed by delays due to rework.
“Our eTMF system’s automated audit trail captures metrics from the day-to-day process of completing clinical trial documents,” Vicari said. “Sponsors and CROs can see how many times documents are sent back and forth from the site to the sponsor for review and approval, and can discern trends to uncover why the process is moving too slowly. It’s a dynamic, in-process approach to TMF management that saves time and improves compliance.”
Advanced Clinical Gets Its TMF in Peak Shape
A recognized market leader, Advanced Clinical has performed more than 250 clinical studies worldwide for sponsoring companies. For nearly a decade, the company relied on common, paper-based systems to collaborate with its customers on various documents but they did not provide real-time visibility, access, or in-process functionality that is so crucial to ensuring speed and compliance. Advanced Clinical searched for a new life sciences-specific solution, began implementation in early 2013, and evolved its processes to fully leverage the new system and adopt a truly active TMF operating model.
The company has since run multiple, global clinical trials and has experienced dramatic improvements in trial efficiency. Now, all trial data and documents are managed continuously in the eTMF for real-time visibility across all stakeholders, including investigators and clinical research partners. Specific workflows automate many of the previously manual steps throughout the study plus the system captures an accurate audit trail for constant status on TMF completeness. There is no need for time-consuming reconciliation prior to archiving documents at study close-out—all materials have already been incorporated in the system throughout the course of the trial.
The Winds Are Shifting Fast
The shift towards paperless technologies and a more active approach to TMF management is gaining momentum. In fact, 61 percent of respondents to Veeva’s TMF survey stated that using an eTMF application improved audit and inspection readiness, and 63 percent believed that managing filing processes in a cloud-based eTMF would shorten clinical development time. Respondents who use eTMF applications reported improved central and remote monitoring of clinical trials (48 percent) as well as better tracking and reporting (45 percent) and greater visibility into performance (42 percent).2
Across the industry, life sciences companies say they not only want new technology, but also to shift their approach. In fact, nearly all 100 global life sciences companies that took part in Veeva Systems’ TMF Maturity Model Assessment program to date said they want a more active operating model. Participants averaged a score of 2.54 on a maturity scale of 1 to 5 where active TMF management starts at 3.0, suggesting that most companies still fall just slightly below this yardstick. One medical device manufacturer, for example, scored a 2.19 when it still relied on a paper TMF but has since elevated to 3.2 on the scale after adopting a new active, eTMF operating model and solution. Advanced Clinical has also increased its rating, and is now certified to conduct the analysis for its customers.
“As a CRO, we wanted a tool that would enable us to objectively demonstrate where our clients stood in terms of their TMF management approach, giving critical context to our recommendations on how we could guide them along the maturity curve,” said Vicari. “We became certified on the Veeva TMF Maturity Model so we can help our customers purposefully undertake this transformation and reap the benefits, well beyond inspection readiness. It offers a competitive advantage to us, but more importantly, to our customers, and is clearly the direction our industry is moving.”
When a life sciences company actively utilizes eTMF technology for clinical trials, its TMF is no longer ignored until the end of trials and, instead, aligns technology with processes—not the other way around. It becomes an integral component of daily clinical operations, helping organizations plan, manage, and execute their development strategy; maximize resources; and, ultimately get innovative drug therapies to patients faster. With an active approach, companies can get the most out of modern technology and be positioned to secure an insurmountable competitive advantage.
*The Veeva 2016 Paperless TMF Survey was released in June 2016 but was not released at the time this article was produced.
References:
- Viglya, as posted on the UK MHRA web site. For more: http://viglya.com/uk-mhra-updated-definition-of-a-critical-gcp-inspection-finding/
- “Veeva 2015 Paperless TMF Survey: Annual Report,” by Veeva Systems, October 2015. For more: http://go.veeva.com/tmfsurvey2015
About Veeva Systems
Veeva Systems Inc. is a leader in cloud-based software for the global life sciences industry. Committed to innovation, product excellence, and customer success, Veeva has more than 400 customers, ranging from the world’s largest pharmaceutical companies to emerging biotechs. Veeva is headquartered in the San Francisco Bay Area, with offices in Europe, Asia, and Latin America. For more information, visit www.veeva.com.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!




