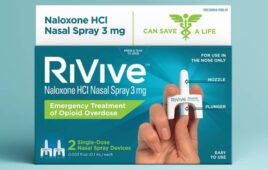
Harm Reduction Therapeutics announced that it received FDA approval for its over-the-counter RiVive naloxone nasal spray. Pittsburgh-based HRT designed the 3 mg naloxone HCl nasal spray for the emergency treatment of opioid overdose. Approval helps make free or low-cost OTC nasal spray widely available in the U.S. The FDA first approved Narcan 4 mg OTC…
Court rejects Johnson & Johnson bankruptcy gambit over talc suits
A U.S. bankruptcy judge in New Jersey has dismissed a Johnson & Johnson-created company’s bankruptcy filing to resolve talc-related lawsuits. Judge Michael Kaplan’s ruling on July 28 was similar to a U.S. Court of Appeals for the Third Circuit ruling months ago, which failed to find immediate financial distress over the lawsuits. As recounted in…
Timeline: Navigating Johnson & Johnson’s talc lawsuits and their stock performance impact
Johnson & Johnson continues to face legal challenges over allegations that its talcum powder causes cancer. Recently, a judge dismissed the company’s latest attempt to settle thousands of lawsuits through bankruptcy, marking the second time J&J’s bankruptcy strategy has been rebuffed. We take a look at the timeline of the talc litigation below. Johnson &…
Astellas latest to wage war against the Inflation Reduction Act
In a recent move, Astellas Pharma challenges IRA, a step that sees them join other pharmaceutical heavyweights in a lawsuit against the federal government. The bone of contention is the Inflation Reduction Act (IRA), a law primarily designed to drive down prescription drug prices, a proposition that no Republican supported. Astellas, along with the other…
The recent FDA thumbs-up for Beyfortus could kickstart a new chapter in RSV prevention
Respiratory syncytial virus (RSV) is a common cause of severe lower respiratory tract infections like pneumonia and bronchiolitis in infants and young children. Traditionally there have been no approved treatments for RSV infections, with care being largely supportive. But the landscape is quickly changing. The FDA recently approved Beyfortus (nirsevimab), the first immunoprophylactic to prevent…
Novartis aims to offload eye care unit to Bausch + Lomb for up to $2.5 billion
Novartis has agreed to sell ophthalmology assets to Bausch + Lomb for an upfront payment of $1.75 billion, with potential milestone payments of up to $750 million. The total value of the deal could reach $2.5 billion. Novartis expects the deal to improve their focus more on higher-margin drugs. Under the terms of the deal,…
The economic impact of pharma giants’ fight with the U.S. government over drug pricing reform
Big Pharma firms Merck and Bristol Myers Squibb are challenging President Biden’s Inflation Reduction Act, alleging the law’s plan to lower drug prices infringes on their rights. The U.S. Chamber of Commerce — whose members include drugmakers AbbVie and Eli Lilly — is also aiming to block Medicare from rolling out the program. In 2022,…
Exploring Merck’s key arguments in its constitutional challenge against the Inflation Reduction Act
In a strong move against the federal government, Merck & Co., based in Kenilworth, New Jersey, has launched a lawsuit against the Inflation Reduction Act (IRA). The firm has retained the services of prominent law firm Jones Day, at times known for its assertive litigation style. In recent years, the firm has also been involved…
The need for a data-driven culture in life sciences: What you don’t know can hurt you
Preventing sudden failures and unexpected downtime in life sciences organizations starts with promoting a data-driven culture across the enterprise. Promoting a data-driven culture across the enterprise is what makes today’s most successful life sciences organizations stand out. Efficient operations are only possible when the processes underlying those activities are functional, and this type of reliable…
The economics behind the Adderall shortage: Why low prices lead to scarcity
On October 12, 2022, the FDA confirmed what many patients with attention-deficit / hyperactivity disorder (ADHD) already knew: There was a shortage of the immediate-release formulation of amphetamine mixed salts commonly known by the brand name Adderall. The implications of this announcement have rippled across the United States, affecting scores of patients reliant on the…