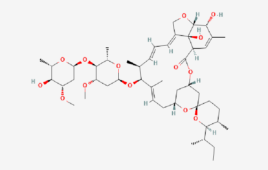
The FDA has pulled the emergency use authorization (EUA) for bebtelovimab, the monoclonal antibody from Eli Lilly (NYSE:LLY), because it no longer adequately protects against the BQ.1 and BQ.1.1 omicron subvariants. In late November, the BQ.1 variant was responsible for 27.9% of U.S. COVID-19 infections, according to CDC. The BQ.1.1 variant represented 29.4% of infections. Meanwhile,…
Study finds no connection between COVID-19 vaccine and shingles
After the introduction of COVID-19 vaccines, anecdotal evidence linked the administration of the vaccines with herpes zoster (shingles) infection. A new study published in JAMA Network Open, however, debunks that assertion. Reviewing data from more than two million U.S. recipients of COVID-19 vaccine, the study authors concluded that the incidence rate ratio of shingles following COVID-19…
AstraZeneca abandons plans to launch Vaxzevria COVID-19 vaccine in U.S.
AstraZeneca (LON:AZN) was among the first companies to develop a COVID-19 vaccine, but the firm has given up on marketing its Vaxzevria vaccine in the U.S. The company’s CEO, Pascal Soriot, announced that it had asked the FDA to withdraw its regulatory paperwork for Vaxzevria. The vaccine developed jointly with Oxford University saw considerable success…
Pfizer beats The Street in Q3, raises guidance
Pfizer (NYSE:PFE) shares ticked up before hours on third-quarter results that topped the consensus forecast. Shares of PFE ticked up 3.7% at $48.29 apiece before the market opened today. The New York-based pharmaceutical giant posted profits of $8.6 billion. That amounts to $1.51 per share on sales of $22.6 billion for the three months ended…
Biden urges Americans to get updated COVID-19 bivalent booster
After receiving a COVID-19 bivalent booster on television, President Biden asked all eligible adults to receive their booster to help ward off a potential uptick in infections in the coming months. The updated vaccines from Pfizer/BioNTech (NYSE:/PFE, Nasdaq: BNTX) and Moderna (Nasdaq:MRNA) are “incredibly effective,” Biden said. “But the truth is, not enough people are…
Ivermectin fails to match placebo in ongoing COVID-19 study
Participants in a Phase 3 COVID-19 study who received the antiparasitic ivermectin fared about as well as those who got a placebo. Ivermectin recipients recovered in a median time of 12 days, while placebo recipients convalesced in 13 days on average. The data from the ongoing ACTIV-6 study were published in JAMA. The study authors concluded that ivermectin did…
Developers of Pfizer/BioNTech COVID-19 vaccine to establish Asia-Pacific mRNA R&D center
mRNA Victoria announced today that the developers of the Pfizer/BioNTech COVID-19 vaccine will establish a clinical R&D center in the Asia-Pacific region. The center enables Victorian and Australian researchers to advance the next generation of mRNA vaccines and therapies. The R&D center aims to facilitate partnerships on scientific mentoring, business development advice and drug design.…
Pfizer and Moderna seek FDA nod for omicron-adapted COVID boosters in children
Pfizer/BioNTech (NYSE:/PFE, Nasdaq BNTX) and Moderna (Nasdaq:MRNA) have both won emergency use authorizations in the U.S. for bivalent vaccine boosters based on BA.4/BA.5. Pfizer is aiming to win authorization to use its updated COVID-19 vaccine in children aged 5 through 11 years. Moderna has requested that FDA greenlight the use of its omicron booster in…
Pfizer to provide up to 6 million Paxlovid courses to developing countries
Pfizer (NYSE:PFE) has reached an agreement to supply as many as six million courses of its oral COVID-19 antiviral Paxlovid (nirmatrelvir and ritonavir) to the Global Fund, which seeks to help more than 100 low-to-middle income countries fight diseases such as COVID-19, HIV, tuberculosis and malaria. Pfizer notes that the agreement with the Global Fund is…
FDA updates ad comm meeting date for sabizabulin in hospitalized COVID-19 patients
The oncology biopharma Veru (Nasdaq: VERU) has announced that the FDA has moved the date for its Pulmonary-Allergy Drugs Advisory Committee to discuss the potential emergency use authorization (EUA) of sabizabulin for hospitalized COVID-19 patients. The meeting is now slated for November 9, 2022. It was originally scheduled for October 6, 2022. VERU shares skidded…