Bausch + Stroebel participates in an exclusive Q&A on their 2016 INTERPHEX Exhibitor Award: the Biotech Innovation Award.
On April 26, 2016, the first day of INTERPHEX, six winners were announced for the competitive INTERPHEX Exhibitor Awards. The six categories and the corresponding companies who won the awards are as follows:
- Best in Show: GE Healthcare Life Sciences
- Best New Product/Service: Nemera
- Editor’s Choice: Catalent Pharma Solutions
- Best Technological Innovation: Videojet Technologies
- Efficiency Champion [NEW]: Millipore Sigma
-
Biotech Innovation Award [NEW]: Bausch + Stroebel
Two representatives from Bausch + Stroebel—Katrin Schuler, Business Development, and Jim Nadlonek, Pharmaceutical Operations Specialist—participated in an exclusive Q&A with Pharmaceutical Processing to talk about their INTERPHEX Exhibitor Award, the Biotech Innovation Award. Their edited responses are below.

Q: Congrats on your Biotech Innovation INTERPHEX Exhibitor Award! Could you tell us a little bit about VarioSys®?
A: The VarioSys® was developed to support a request from Boehringer Ingelheim (BI). In 2012/2013, BI approached Bausch+Stroebel and SKAN to develop a filling system that was highly flexible to fill vials, syringes, and/or cartridges that was a space savings unit with the line inside an isolator (providing the highest sterility assurance). In addition, the line had to be cost effective and modular to enable expansion as technologies develop.
SKAN Inc. provided the isolator and Bausch+Stroebel provided portable filling skids. The first two skids developed were the syringe filling skid and vial filling skid. When the cartridge configuration is available in a pre-sterilized tub configuration, we will be ready. We can already process a cartridge in the VarioSys® by using a washer and tunnel. The line design can process ready-to-use (RTU) containers or we can process containers the traditional way or bulk process. We offer a suitable configuration to meet the needs of the client as well as flexibility and modularity.
Q: How long has VarioSys® been commercially available?
A: The syringe filling skid was sent to BI in the spring of 2014 and the vial filling skid was sent in the summer of 2015. BI wanted to stagger the delivery to support the implementation and validation schedule.
Q: What sparked the idea behind VarioSys®? Any interesting discoveries along with way?
A: The spark was generated by BI and Bausch+Stroebel, and SKAN placed on their thinking caps to take the line from a discussion to concept and fabrication and finally implementation. The collaboration with all three companies was the starting point. Bausch+Stroebel and SKAN have worked on many projects over the years and we worked the concepts with BI to bring the line to the final production.
As always, there are some technical issues and more importantly product issues one has to support. Working with vapor phase hydrogen peroxide (VPHP) and the regulatory pathways need careful discussions. At first, the process was to pass the pre-sterilized tubs of syringes or vials through an airlock. This presented a decision to ensure the regulatory pathway was efficient and effective without raising any concerns. We switched from the airlock to an effective way to pass the tubs into the system without VPHP contact. The change impacted the design group at a very critical phase of the project.
Q: How does the design of VarioSys® differ/compare to its competitors?
A: The VarioSys® was the first flexible and modular unit. The SKAN PSI-L unit provides the modulator to add isolators, while Bausch+Stroebel would insert the different technologies onto the portable filling skids to provide the flexibility. With this concept, one can fill a variety of containers in one filling suite, compared to having several filling suites with different equipment to support different containers. We have so much flexibility we can even expand the line by adding your typical washer and dry heat tunnel to the front end of the line to make it more efficient for medium batch sizes.

Q: Were there any challenges you faced in the design and creation of VarioSys®?
A: Managing the space and managing the cost was the key challenge with the first line. To keep the units cost effective, one must avoid doing customization the system or module. SKAN provided the standard PSI-L unit and B+S had to work within the framework of the standard unit. B+S then took our syringe model SFM platform, the vial model KSF platform for filling and stoppering, and the vial sealing model KS platform for sealing. Modernized the platforms and made them fit on the footprint allowed by the framework of the SKAN isolator. During the INTERPHEX event, specifically during the IPS Aseptic Technology Tour, we discussed the “Flexible Revolution and Evolution – VarioSys®.” At this time, keeping up with the evolution with the technology we can place in the unit or in front or on the sides of the modular system is even more taxing and challenging.
Q: What, would you say, are one of the key market needs that VarioSys® addresses?
A: This question is spot on when you ask what the system addresses! The first word that comes to mind is line addresses many needs. We have clients that purchased the system to fill clinical and commercial products on the same line. We have clients making the unit the “Centre of Excellence” with the technologies. Clients are dropping the units into emerging markets around the world to meet increasing requirements to perform more and more work in the region. The unit is ideal for Antibody Drug Conjugates (ADC) to handle potent products and where small batch sizes fit perfectly into the line.
We have a client manufacturing personalized medicines (patient specific) in the best possible environment for safety and speed in sending the drug back to the patient. Biotech product batch sizes are smaller than the typical small molecule large batch sizes. CMOs are demanding more and more flexibility. You can start off with one SKAN isolator and a vial filling line (fill, stopper, and seal) one year and add syringes or lyo capabilities in 2 years, 3 years, or beyond.
Finally, recent years have been focused on the activities at compounding pharmacies. The VarioSys® addresses the issues surrounding this industry very well. To say the least, the list is fairly endless, hence evolution continues as we provide solutions for our clients.
Q: I see that VarioSys® is broken down into a standard isolator (by SKAN) and machine modules (by Bausch + Ströbel). Could you explain how that works and how the two technologies work together?
A: In some respect, the SKAN units are providing the isolator module or provide the modularity. SKAN can build the system with 1, 2, 3, or more isolators, hence the modularity. Bausch+Stroebel provides the flexible portable filling skids. The biotech and pharmaceutical industry understands skids and we are providing the portability of the filling system on a skid. That being said, we use “lock-and-key” or “plug-and-play” interchangeably. We roll our technology into the isolator, plug in the power and utilities, and you are ready to begin. The seal around the filling skid is the same technology used to seal the windows on standard isolators. Think of it this way: SKAN provides the surrounding environment suitable for aseptic processing and we provide technology that is placed into the environment. Also note, we have added the GEA lyophilizer into the program and our Excellence United partner, Harro Hofliger, can provide I.V. bag filling and robotic handling and pipetting on the portable skid.
Hence, you see this in our literature—the four companies are working together to add technologies and to address the needs for the industry.

Q: On your website it says that VarioSys® expandable. Could you explain why that is?
A: Pictures sometimes make this easier to explain how the unit is modular and expandable. Below you see a rapid transfer airlock with one SKAN module and the unit has one vial filling line. As you add technologies, you can add the second SKAN unit to make the line more efficient. The second unit enables you to add space for handling the trays or tubs in a larger area or we add technologies. Another thing to note is the filling line in the first pictures fits into the second unit in the picture. Hence, you do not need to purchase another filling line. One can add at least four units if you wish. We have three units in design and construction to have three modules from SKAN with the middle module containing the lyophilizer from GEA. The first unit had the vial filling and the third unit has the crimping unit. Key to this line under design and construction is we added a vial washer and dry heat tunnel to make the line more efficient.
Q: Could you describe the various pump systems that can be integrated into VarioSys®?
A: This is a great question and a big piece of the evolution for the VarioSys® line. The first line was built with rotary piston pumps. As we were in discussions with other clients, we added other technologies, such as, peristaltic pump(s), time pressure filling, and finally powder filling. We also wanted to make this cost-effective by installing the three liquid filling systems in the first module with the needle holder in the second module for the filling operation. What this does for the client is they only have to purchase the filling system once while the second unit will have the needle holder and different filling technologies.
Q: In general, what are some of the operational best practices for production systems?
A: The best practice is the use of the SKAN isolator technology to meet the regulatory guidance around the world. A recent addition is having the module available in a very good RABS configuration very suitable to meet regulatory guidance around the world. The client has the choice.
For Bausch+Stroebel, our best practice is we are using the same technology on the VarioSys as we would on higher speed lines. This enables a more simplified technical transfer to the higher speed lines. Once you establish the critical product characteristics on the VarioSys you can move the requirements to other lines.
The VarioSys® line is suitable for a wide range of applications from the manufacturing of clinical supplies through commercial production batches all on one line. All FDA and cGMP requirements for the pharmaceutical and biotechnology production are met on the line.
Q: What would you say is one of the greatest advantages of production systems?
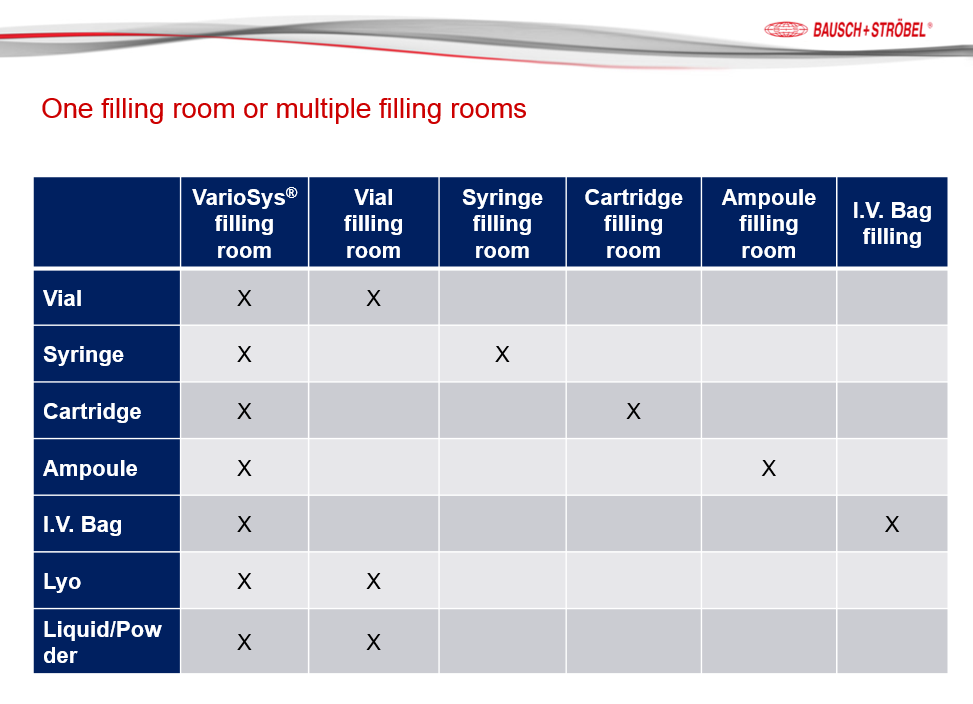
A: For starters, you have the capability to fill many different containers (vials, syringes, cartridges, ampoules, I.V. bags, plus aseptic compounding) in one filling suite, compared to many filling suites with different filling lines for each container plus you can add modules and filling technology as your business case builds over time. You can manage your capital cost over time while at the same time reducing the overall operational costs associated with an ISO 5 filling suite. For example, you can buy one module and a vial filling line in the first year. Two, three, or four years later you can add the lyophilzer or syringe filling skid or any other filling technology as you need it and when you need it.
Furthermore, once qualified, the system is self-contained and easily be re-deployed to different locations, such as, from technical expertise centers to emerging markets or to markets with drug shortages without the typical long qualification and validation processes. The system can be installed in a ISO7 / Grade C clean room with the plug-and-play technology is operational in a short period of time. In fact, one can potentially ship just the filling skid to areas around the world once you have the modular system set up in a particular country or region.
8 advantages of VarioSys®:
- Utmost flexibility to fill a variety of different containers
- Reduced space requirements for the processing area
- Isolator modularity and adaptability for future expansion
- Standardization of portable skids reduces investment costs
- High product safety and reproducibility
- Simple to implement and operate – plug and play
- Rapid changeover (<30 min) from one process to another (e.g. from vial to syringe process)
- State-of-the-art pharmaceutical and biotechnological processing line
Q: What is one of the greatest challenges associated with production systems?
A: The biggest challenges we have is keeping up with our client needs and the evolution of technologies to put on the line. Our designers are busy. During the IPS Aseptic Tour, we showed some of the evolution. It started with a couple of line items in 2012/2013. Each year after, the number of line items increase for each year and for each company.
Q: How do you hope to change the market with VarioSys®?
A: My colleague Thomas Buehler, Sales Group Leader North America for Bausch+Stroebel, provided the quote of the show. We were discussing with a client how to protect technologies on your equipment. We talked about how difficult it is with all the social media methods—someone can record the Web Ex and before you know it the topic is up on the social media. Thomas looked at the client and myself and stated: “The best way to protect your technology is to be innovative and don’t stop being innovative!” Follow that up with the Bausch+Stroebel motto: “Precision to the Highest Standard.”
Q: What was your reaction when you heard that your company had won the award?
A: Mostly, we all had big smiles on our faces! Smiles of pride in what we do and how we do it! We all knew we had something special with the VarioSys® line and our client response has been much better than expected. We will continue to innovate and offer more and more technologies. Our client that purchased the line was at the show was very, very excited! The award did validate the selection of our equipment by the client. The long hours and hard work putting this together was worth every minute!

Q: Beyond the INTERPHEX Exhibitor Awards, what were some of your favorite memories at INTERPHEX 2016?
A: It is like going to Hawaii. They tell you to take a helicopter ride around the island. Once you see the highlights, you can note them down and travel back to the site for more detailed talks. In addition, I tell my clients to send both the junior members and senior members around on the IPS Aseptic Technology Tour or other IPS tours to see the latest cutting edge technologies.
Follow us on Twitter and Facebook for updates on the latest pharmaceutical and biopharmaceutical manufacturing news!



