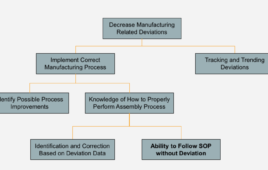
In 2015, 30 percent of the 40 GMP warning letters issued by the FDA for data integrity deficiencies referenced training issues or requirements.1 There are a number of challenges hindering effective training in life sciences. For example, most companies use one system to manage standard operating procedures (SOPs) and other documents, and a different application…