By Sino Biological
Therapeutic monoclonal antibodies have emerged as the fastest-growing class of therapeutics since the mid-1980s from the increasing number of pharmaceutical companies seeking more drug modalities to extend their pipelines. Additionally, biosimilars now have more opportunities to enter the market as patents of some blockbuster monoclonal antibodies begin to expire. Given this context, assessing the safety and efficacy of biotherapeutics has become imperative during the drug-development process. In particular, pharmacokinetic (PK) and anti-drug antibody (ADA) assays are conducted to study and evaluate new drug candidates and biosimilars. For PK/ADA assays, anti-idiotype antibodies (Anti-IDs) are powerful tools to measure the concentration of antibody drugs in patient samples and serve as positive controls for anti-drug antibodies (ADA).
Anti-IDs in Pharmacokinetic (PK) Assays
Pharmacokinetics (PK) describes and characterizes the four different phases of a drug in human or animal body: absorption, distribution, metabolism, and excretion (also known as ADME). In drug development, PK assays provide essential information on a drug’s interaction with the body, as well as the intensity and duration of its efficacy. For the development of biosimilar drugs, comparative PK assays are required to evaluate potential differences in biological activity to the reference drug.
Based on different binding modes and properties (Figure 1), anti-idiotype antibodies can be classified into three types: antigen-blocking, non-blocking and complex specific. Based on these features, different formats of PK assays can be set up to measure free, total, or bound antibody drug concentrations in serum.

Figure 1. Types of Anti-IDs in PK Assays
Anti-IDs can be used to detect and quantitate antibody drug levels in animal or human serum and are critical detection reagents for PK studies. There are a variety of analytical methods for quantitative analysis of antibody drugs, with ELISA as the most commonly used format. In an anti-ID capture ELISA (Figure 2), anti-IDs are coated to a plate and then samples containing the antibody drug are added to the system. The antibody drug is then quantified with labeled anti-IDs that specifically bind to the idiotope of the drug.

Figure 2. Principle of Anti-ID Capture ELISA
Anti-IDs in Immunogenicity/Anti-Drug Antibody (ADA) Assays
Immunogenicity assessment involving the detection of anti-drug antibodies (ADA) is a critical step during the development of therapeutic proteins, such as monoclonal antibodies, ADCs, and fusion proteins. In these situations, a multi-tiered testing approach (Figure 3) is usually employed. The approach utilizes a sensitive screening assay to identify positive antibody samples, a confirmatory assay to minimize false positive results followed by a characterization assay to assess the neutralizing capacity of antibodies.
During the whole process, anti-idiotypic antibodies are indispensable reagents. Different assays can be used to detect ADAs, including ELISA, radioimmunoprecipitation assay (RIPA), surface plasmon resonance (SPR), and electrochemiluminescence (ECL). Among these, bridging ELISA is the most commonly used method that allows detection of all isotypes of ADAs (IgG, IgM, IgA, etc.). In a typical bridging ELISA, the antibody drug is precoated on a plate, and the labeled antibody drug is incubated with patient samples to test the presence of ADAs (Figure 4). Anti-IDs will be used as positive controls or reference standards for qualitative analysis of ADAs in samples.

Figure 3. Multi-Tiered Testing Approach

Figure 4. Principle of ADA Bridging ELISA
Anti-Idiotype Antibody Generation Packages
In order to speed up your drug development, Sino Biological provides a full range of custom anti-idiotypic antibody production services from antigen preparation, anti-idiotype antibody development to detection method establishment and kit development. As well as a team with strong expertise and experience in targeting diverse modalities, including full-length mAb, F(ab’)2 , Fab, scFv, VHH, ADCs, bsAbs, and Fc-fusion proteins. Customized anti-idiotypic antibodies can be used to set up pharmacokinetic (PK)/ADA assays to determine specific antibody drug or ADA levels in samples.

Table 1. List of Anti-Idiotype Antibody Services
Case Study #1: Development of Anti-IDs Targeting BsAbs
Bispecific antibodies (BsAbs) are genetically engineered antibodies with two specific antigen-binding sites that recognize two different epitopes or antigens. Compared with monospecific antibody drugs, BsAb drugs often have an increased tendency for proteolysis, aggregation, physical instability, etc. Also, the binding of two different antigen-binding sites to their corresponding ligands may create large steric hindrance effects on each other. Considering these factors, it’s very challenging to screen anti-IDs against different antigen-binding epitopes of bispecific antibodies.
Understanding those obstacles, Sino Biological has successfully completed several anti-ID development projects that recognize BsAb drugs. These generated anti-IDs can bind to two different antigen-binding sites respectively and with great specificity. As shown in Figure 5, seven anti-idiotype mouse monoclonal antibody clones showed highly specific binding to the BsAb drug, and exhibited no binding to human IgG isotype control or total human IgG. In addition, we also screened a panel of neutralizing and non-neutralizing anti-IDs against monospecific unit A and B of BsAb drugs. Antibody pairing was then performed in capture ELISA format. The detection sensitivity reached pg level and the recovery rate was 80-120% in different concentrations of human serum. Related data are listed in the following Figure 6 and 7.

Figure 5. Binding Specificities of Anti-ID mAbs

Figure 6. Standard Curve and Recovery of Anti-ID mAb Pairs against Unit A
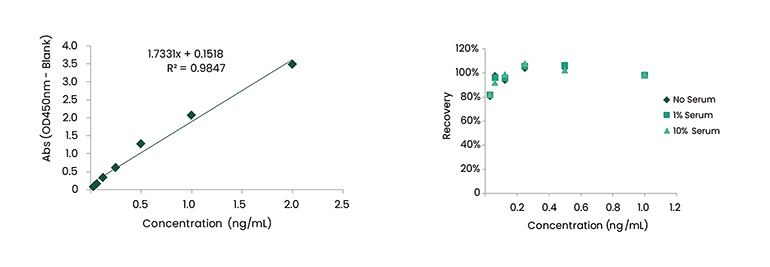
Figure 7. Standard Curve and Recovery of Anti-ID mAb Pairs against Unit B
Case Study #2: Generation of Anti-IDs Targeting scFv/VHH
scFv is a fusion protein of the immunoglobulin heavy and light chain variable regions, which are connected by a peptide linker. VHH is a single variable domain located on a heavy chain, also known as a nanobody. Features of scFv and VHH include a small size, great specificity, strong tissue penetration, and high stability. However, due to their low immunogenicity, the development of anti-scFv/VHH antibodies remains challenging.
To address this technical challenge, Sino Biological established the scFv/VHH immunization platform. The success rates of immunizing mice with scFv and VHH reach 80% and 100%, respectively. We also launched a rapid immunization protocol for VHH rendering it possible for mice immunization to be completed in 37 days. In the Figure 8, the titers of VHH mice are sufficient to use for subsequent cell fusion, and rapid immunization has shown comparable titer levels in contrast with routine immunization.

Figure 8. Titer Check of VHH Immunized Mice
Sponsored content by Sino Biological





Tell Us What You Think!