Some 1.8 million people acquire HIV each year, with the World Health Organization estimating that one in five people living with HIV do not realize they are infected. Therefore, strategies for faster and earlier diagnosis are as important as ever to limit the spread of HIV.
Currently, the most common test for HIV looks for an immune response by looking for the antibodies the body produces in response to infection. Typically, a blood sample is taken from the patient and then sent to a laboratory for this test, which takes around a week. All positive results are then confirmed by a second test.
Alternatively, in resource limited settings where the infrastructure is not available to conduct such tests, lateral flow devices (resembling at-home pregnancy tests) can be used to make an initial diagnosis.
The problem with testing for antibodies (the immune response) is that it can produce false-negatives when the test is conducted before the immune response has occurred. It takes around four weeks for the body to generate antibodies to HIV, called the ‘window period’, therefore tests cannot be carried out before this time. The advice is to wait three months to take an HIV test.
The most rapid and reliable way of detecting HIV is to search for the HIV genetic material directly in blood, using a process called PCR (polymerase chain reaction). This has the advantage of not needing to wait for such a long time after exposure, being used after only three days.
Traditionally this process, known as molecular diagnostics, has been performed in a laboratory by trained personnel. Recent technological advances have however brought the possibility of conducting molecular diagnostics out of the laboratory and next to the patient at the point-of-care.
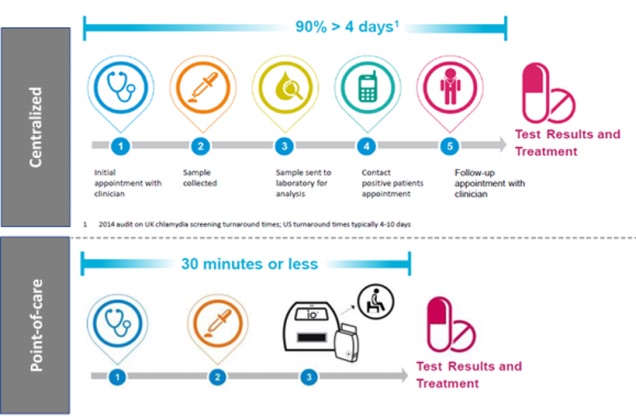
Timescales for the diagnosis of a sexually transmitted infection, chlamydia, via centralized and point-of-care testing (Source: Atlas Genetics).
This is part of a wider trend in the field of biosensors which is undergoing a shift to the point-of-care, a sector estimated to be worth ~$33 billion by 2027. Point-of-care testing can be defined as a test which takes under an hour on a portable device (IDTechEx Research). This means that samples no longer have to be taken and sent to a laboratory for analysis, and then the results fed back for follow up treatment.
Point-of-care testing has particularly high value in the diagnosis of sexually transmitted infections, where a rapid diagnosis can demonstrably reduce the spread of infection. Numerous studies conducted in the US estimate that ~40 percent of all patients who present at a sexual health clinic are not contactable once they leave, meaning that the spread of infection continues—even among those who are seeking diagnosis and treatment.
With the advent of point-of-care molecular diagnostics, tests for infections such as HIV and other infectious diseases can be carried out while you wait. Treatment can therefore be given immediately if required, greatly reducing the likely spread of infection.
The IDTechEx Research report Biosensors for Point-of-Care Testing 2017-2027 gives an analysis of the trends in the field of medical biosensors, and lists the new technologies and devices which are likely to be highly disruptive to the in vitro diagnostics market.
(Source: IDTechEx)




